Citation: Pizitz T, Mealing D, “When Every Second Counts…”. ONdrugDelivery Magazine, Issue 102 (Nov 2019), pp 24-25.
Todd Pizitz and Donald Mealing look at efforts to reduce the number of opioid-related deaths in the United States, and introduce a nasal device that delivers naloxone..
In emergency medicine, seconds can be the difference between life and death. The average response time for emergency medical responders is estimated to fall between eight and 14 minutes, with rural areas having longer wait times.1 When first responders are not emergency medical personnel, having immediate access to rescue medication becomes a necessity to reverse the effects of an opioid overdose.
“Mishandling, or easy access to opioids in a household, have led to an increase in overdose reactions.”
According to the US Centers for Disease Control and Prevention (CDC), more than 47,000 people died from opioid-related deaths in the US in 2017. The estimated death rate of 130 people daily due to opioid overdoses continues without much relief. In fact, accidental ingestion of opioid medication resulting in an overdose death accounts for an estimated 40% of all the opioid overdose deaths.2 This includes family members of a patient with an opioid prescription.
Mishandling, or easy access to opioids in a household, have led to an increase in overdose reactions. Khan, Bateman and Landon3 found that individuals with family members who had been prescribed opioid medication were nearly three times more likely to report an opioid overdose that resulted in a hospitalisation compared with individuals whose family members were not receiving any sort of opioid prescriptions.
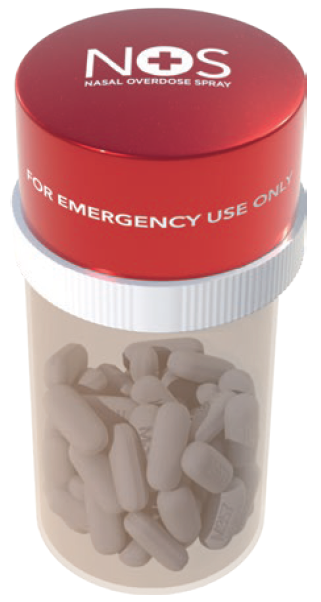
Figure 1: The CounterAct Cap.
The CDC calculates that nearly 200 million opioid prescriptions are written annually and that number has been slowly decreasing. Efforts to reduce the opioid crisis have included reducing the number of opioid prescriptions written, educating the public about the dangers of opioids, and increasing the availability of opioid reversal medication Naloxone. The US Surgeon General even recommended the public carry naloxone to help reduce the deaths associated with opioids.
Naloxone is marketed as a generic medication to treat opioid overdoses, and brand names such as Narcan and Evizo have emerged as US FDA-approved naloxone-based medications to counter the effects of an opioid overdose. Unfortunately, although these two FDA-approved formulations of naloxone are available for public use, the overdose death rate from opioid overdoses has not significantly declined.
Efforts to make naloxone more available have emerged from the US legislative arena, with lawmakers pushing for co-prescription laws mandating prescribers who write opioid medications to also consider prescribing naloxone formulations and mandating the co-prescription if any of the three co-prescription criteria are present:
- A patient receives a prescription for an opioid medication that has a 90 mL morphine equivalent
- The prescriber suspects a potential for abuse of the opioid medication or the patient has a history of substance abuse
- The opioid medication is also prescribed with a benzodiazepine.
In September 2018, California passed a co-prescription law mandating prescribers to co-prescribe naloxone with opioid medications. Other states are following the same pathway to co-prescription of naloxone with opioids.
To increase speed of access and wider availability of naloxone, the CounterAct Cap was designed (Figure 1). It places a single-unit-dose of Naloxone on top of the container holding an opioid patient’s prescription pills. In a suspected overdose emergency, a patient’s family members, friends or associates can instantly administer the naloxone spray after calling 911, thus saving precious time.
The safety cap twists off to reveal a folded nozzle that is easily extended. Once the nozzle is fully extended, it can be placed into the overdose victim’s nose and the spring-loaded trigger can be pressed to instantly release a 4 mg dose of naloxone to the victim (Figure 2). The idea behind the CounterAct Cap was also that having the two medications paired together would act as a reminder of the necessity of prescriber compliance – and that mismanagement and abuse of opioid medication can lead to death.

Figure 2: The spring-loaded trigger can be pressed to instantly release a 4 mg dose of naloxone to the victim.
The specific features of the CounterAct Cap include an integrated system of opioid medication housed in a pill container and a 4 mg dose of naloxone located on top inside the CounterAct Cap. The bright-red colour of the cap is designed to help with visually locating the cap. CounterAct has also developed a Smart Cap that has a connectivity component to increase the likelihood of locating the naloxone formulation when an opioid overdose has occurred.
CounterAct is at the beginning of its FDA 505(B)(2) regulatory path as a drug-device combination product. The company has been collaborating with major drug and device manufacturers and investors to bring the CounterAct Cap to market.
REFERENCES
- Howard K et al, “Emergency Medical Services Response Times in Rural, Suburban, and Urban Areas”. JAMA Surg, 2017, Vol 152(10), pp 983-984.
- Khan N, Bateman B and Landon J, “Association of Opioid Overdose With Opioid Prescriptions to Family Members”. JAMA Intern Med, 2019, Vol 179(9), pp 1186–1192.
- “Mortality in the United States, 2016”. NCHS Data Brief No 293, December 2017.

