To Issue 151
Citation: Esser Wiesemann C, “YpsoDose – the Clinic-Ready 10 mL Patch Injector.” ONdrugDelivery, Issue 151 (Sep 2023), pp 6–9.
Christian Esser Wiesemann provides the latest insights into the prefilled and pre-assembled YpsoDose patch injector, which is now available for clinical trials.
INJECTION TRENDS AND TARGET THERAPY AREAS
Subcutaneous injection has emerged as a prominent method of drug administration, with one-third of all injectable agents currently in development falling into this category. In recent years, there has been a noticeable shift towards fixed-dose regimens for antibody-based drugs, followed by an increase in subcutaneous dosing intervals towards once every four weeks, or even less frequent.
“Total number of use steps plays a critical role in achieving a successful injection in the homecare setting.”
Therapy areas are developing in different ways regarding the use of patch injectors (Figure 1), such as highly potent subcutaneous oncology drugs, which require pre- and post-medication along with surveillance both during and after the injection in a hospital setting, and are often administered by trained personnel with a syringe. In autoimmune and rare disease therapies, there are examples of extending to new indication areas for existing drugs, which lead to a higher administered volume in the already established homecare setting, as is the case with AbbVie’s Skyrizi (risankizumab).

Figure 1: YpsoDose® patch injector.
These transitions have sparked discussions regarding the administration setting, and which devices are recommended for at-home care versus hospital settings, including the choice between on-body devices, autoinjectors or manual injection with syringes as the preferred routes of administration.
“Ypsomed and SCHOTT Pharma have collaborated closely at their Swiss development and manufacturing sites to develop the 10 mL cartriQ®.”
RECENT STUDIES ON WEARABLE PATCH INJECTORS
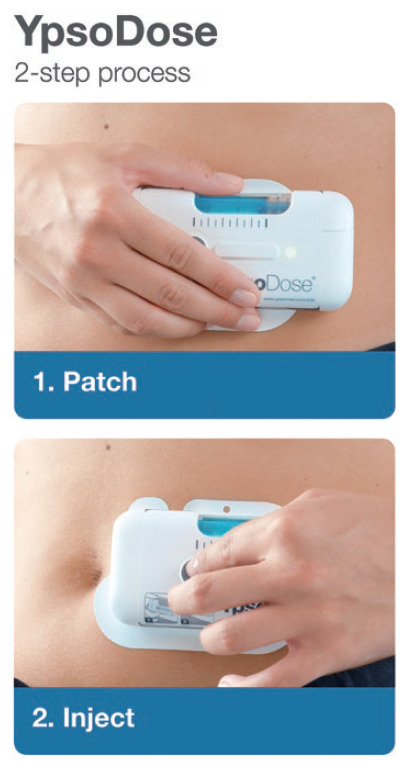
Figure 2: YpsoDose uses a two-step approach – “patch” and “inject” – to reduce user error.
Studies have shown that the total number of use steps plays a critical role in achieving a successful injection in the homecare setting.1 Fewer use steps enable patients to use the on-body device for treatment and perform a worry-free injection, whereas more use steps increase the incidence of user errors.
The patient preference between autoinjectors and on-body devices was tested in a study conducted by Ypsomed. It revealed that a reduction in injection frequency (biweekly to monthly to quarterly) increased the likelihood that patients would prefer wearable on-body devices to handheld autoinjectors. In addition, decreasing the injection duration of the on-body device (30 minutes to 8 minutes) increased the patient preference for the wearable on-body device.2
In another study,3 participants successfully completed simulated injections for the YpsoDose patch injector. The device received high ratings for wearability and usability, regardless of disease state, age or body size. Summarising the trends described above (less frequent, fixed dose, indication extension of existing drugs), a positive effect was seen from the use of on-body devices, which are designed to be patient centric for use in an at-home setting and mitigate the risk of potential misuse for patients who struggle with too many use steps (Figure 2).
PATIENT-CENTRIC DESIGN IMPROVES THERAPY OUTCOMES
YpsoDose is supplied to the patient or healthcare provider (HCP) prefilled and pre-assembled to minimise the number of use steps and reduce the risk of misuse resulting in the handling steps. The pre-assembly of the ready-to-use (RTU) cartridge in YpsoDose is achieved using three sterile barriers protecting the skin needle, cartridge needle and cartridge stopper. All three sterile barriers are removed when the patch liner is removed immediately prior to placement on the skin and the start of injection.
The use of a conventional electromechanical syringe drive mechanism allows YpsoDose to be positioned in any orientation, enabling the patient to choose the most comfortable placing on the desired injection site. The drive mechanism and related software algorithm are designed to modify the injection rate if the patient does not allow the drug to warm up to room temperature. There is also the option for the user to interrupt the injection, due to pain, for example, if this provides an overall advantage in terms of usability for the therapy in question.
STATE-OF-THE-ART RTU CARTRIDGE MAINTAINS DRUG STABILITY

Figure 3: 10 mL cartriQ® from SCHOTT Pharma.
Ypsomed and SCHOTT Pharma have collaborated closely at their Swiss development and manufacturing sites to develop the 10 mL cartriQ® (Figure 3). The 10 mL cartriQ® is the perfect fit for YpsoDose, as it has unmatched inner surface properties (20% lower limit for hydrolytic resistance) and enables low levels of free silicone due to the state-of-the-art baked-on siliconisation process. Furthermore, the 10 mL cartriQ® nest offers 33% higher filling speed compared with other 10 mL cartridge offerings, enabling more efficient processing on fill-and-finish lines.
The cartriQ® sterile barrier crimp cap plays a central role in maintaining the sterility of the RTU cartridge within the pre-assembled YpsoDose device. The cartriQ® sterile barrier is applied during the cartridge assembly and packaging process on SCHOTT Pharma’s new RTU cartridge manufacturing line in Switzerland.
INDUSTRIALISING YPSODOSE – READY FOR CLINICAL TESTING
Ypsomed has implemented cleanroom manufacturing at its Swiss facilities for both the sterile fluid path, which is an integral part of the YpsoDose needle unit, and the sterile barrier crimp cap (Figure 4). The needle unit is ethylene oxide sterilised and the 10 mL cartriQ® is steam sterilised during their respective manufacturing processes at Ypsomed and SCHOTT Pharma. Sterility testing of the final assembled device has been completed internally and in collaboration with pharma clients during feasibility and clinical work.

Figure 4: Cleanroom production of the needle unit.
YpsoDose device assembly is performed using a dedicated line in a clean device assembly environment in Switzerland. Assembly is co-located with Ypsomed’s insulin pump manufacturing, which includes similar processes designed for electromechanical technologies. Dedicated design verification equipment has been developed and implemented at Ypsomed’s testing laboratories in Burgdorf (Switzerland) to facilitate the successful performance of ISO 11608 testing for multiple customers. The cleanroom and device assembly infrastructure are designed to serve multiple clinical trials as well as low-volume commercial quantities (Figure 5).

Figure 5: YpsoDose device assembly.
“Ypsomed is committed to the successful commercialisation of YpsoDose as a new state-of-the-art patch injector.”
Fill-finish contract manufacturing organisations (CMOs) have performed filling batches with the 10 mL cartriQ® format on their filling lines and both SCHOTT Pharma and Ypsomed provide open and co-ordinated support to these companies and their pharma clients. Final assembly equipment is specified with leading suppliers and a first clinical/commercial line will be placed at a leading CMO.
YPSODOSE – THE LATEST SELF-INJECTION PRODUCT PLATFORM
For pharma companies to consider and invest in patch injectors, they need to be able to access reliable device technology and implement standard filling and final assembly processes that provide a turnkey solution that fulfils the needs of pharma companies, HCPs and patients. Fulfilling these requirements with well-thought-out device technology will allow the patch injector market to grow over the coming years and become established as the third self-injection device class, complementing the maturing markets for pen injectors and autoinjectors. The 10 mL YpsoDose has undergone thorough internal testing and comparative studies with pharma customers and Ypsomed is committed to the successful commercialisation of YpsoDose as a new state-of-the-art patch injector.
Find out more about YpsoDose at: yds.ypsomed.com/en/injection-systems/wearable-injectors.
REFERENCES
- Loftus EV Jr et al, “Efficacy, Safety, Patient Experience, and Tolerability of Risankizumab Administered by On-Body Injector for Moderate to Severe Crohn’s Disease”. Adv Ther, 2-13 Vol 40(5), pp 2311–2325.
- Schneider A et al, “Understanding patient preferences for handheld autoinjectors versus wearable large-volume injectors”. Expert Opin Drug Deliv, 2023, Vol 20(3), pp 273–283.
- Lange J et al, “Formative Study on the Wearability and Usability of a Large-Volume Patch Injector”. Med Devices (Auckl), 2021, Vol 14, pp 363–377.

