To Issue 141
Citation: Tunkel M, Gross C, “Patient Journey as the Foundation for Holistic Connected Device Development”. ONdrugDelivery, Issue 141 (Dec 2022), pp 40–44.
Mark Tunkel and Cécile Gross consider the development of connected drug delivery devices and identify how understanding the patient journey from diagnosis to treatment can be used as an early-stage decision-making tool.
AN INCREASINGLY COMPLEX LANDSCAPE FOR CONNECTED DEVICES
The methods available to pharmaceutical companies and device suppliers to support the patient journey and experience offer more possibilities than ever before. Traditionally, the focus of the patient experience has been on optimising the “administration task” as outlined in the instructions for use, with an emphasis on the reduction of use error risk. However, as clinical outcomes increasingly drive payer decision making, developers are expanding their efforts to support the patient journey more broadly. This is being achieved through enhanced methods of training, value-added packaging and longitudinal methods of engagement that span from the onset of a disease state through various life stages.
“Virtually every developer in the branded and generic space is formulating some variation of a digital health approach.”
In addition to these measures, virtually every developer in the branded and generic space is formulating some variation of a digital health approach. Often, this includes add-ons or accessories that are compatible with a commercially available device and provide users with device performance feedback – such as when an autoinjector is ready to inject or when an injection is complete. They can also provide error correction and other means of feedback to help eliminate use errors. The SmartPilot accessory from Ypsomed is an example of a device providing this level of functionality.1
These devices are very effective at helping to mitigate potential use errors with more mature devices. These devices also feature a mobile app that can collect and curate use information for potential integration into a patient’s health plan.
There has also been convergence within the drug delivery ecosystem, where all aspects of managing a disease state are integrated through connectivity that often requires partnerships to address broader steps in the patient journey to leverage complementary technologies. A good example of this is the partnership between Abbott Laboratories’ Diabetes Care division and Novo Nordisk that gathers and transmits insulin dose data from Novo Nordisk connected pens directly into the Abbott Diabetes Freestyle Libre sensor-based glucose management technology to allow patients to manage their disease states more holistically.
All these ecosystems are reliant upon sensor technology and mobile apps that act as the primary interface for all the component pieces. Similar to the add-ons scenario, these ecosystems are addressing known workflows and device component constraints and are beginning to be developed into the devices themselves, which will mark the next phase of electronics and connectivity in drug delivery devices (Figure 1).
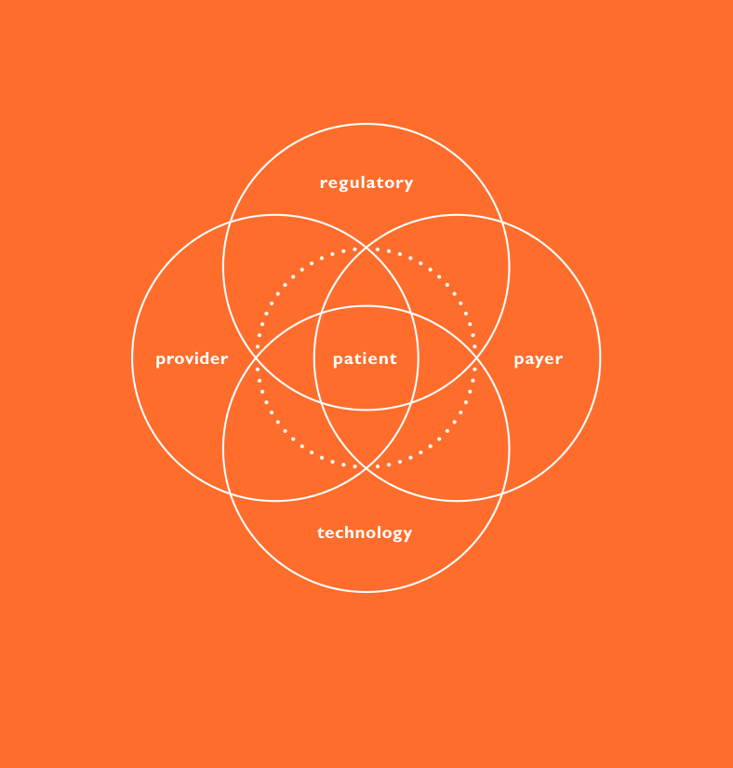
Figure 1: Combination product ecosystem.
THE PATIENT JOURNEY AS THE FOUNDATION FOR INTEGRATING CONNECTIVITY
Achieving success requires establishing a broad baseline of patient needs. A critical tool in helping developers formulate a user-driven strategy is a thorough mapping of the patient journey – to develop an intimate understanding of foundational user needs so that the entire journey is considered at the earliest stages of development.
A comprehensive understanding of the user enables the team to determine how these needs can be most effectively addressed, whether through the device, a software capability or with an interplay between the two augmented by other assets. This approach helps ensure developed technologies are not only meeting actual market needs but doing so in an optimal way – eliminating potential redundancies and conflicts that are inevitable when developing a device and software in isolation.
Understanding, characterising and prioritising user needs is best achieved through applied ethnography, which consists of a combination of interviews and observation within the context of use. This method can yield a deep, longitudinal understanding of the patient journey from diagnosis to treatment selection, onboarding and ongoing use. With an emphasis on system touchpoints like education/training materials, secondary packaging and device interaction, the patient journey can then be leveraged as a critical early-stage decision-making tool.
Nemera’s design research team at its Insight Innovation Center uses a method called applied ethnography to achieve this goal. This relies on interviews and in-context observations of practices, processes and experiences within the patient’s home or use environment. Potential use cases are looked at broadly beyond the administration event or complying with instructions for use. This starts from when a patient is diagnosed, to receiving their device, through the entire process of preparing, administering and disposal, and the times in between treatment to understand how the process changes over time and how frequency of administration may impact the patient experience. It is equally important to gain an understanding of the experience of healthcare professionals to consider relevant settings in clinical environments. This is important in applications where care is provided in both in-home and clinical environments as well as a migration of care, for example, from an oncology ward with significant support systems to an environment of self administration where clinical personnel are not present and the burden of support falls to a family member or caregiver (Figure 2).

Figure 2: Contextual inquiry.
Like drug discovery, this type of research should be started as early as possible in the development process – user needs are the cornerstone in defining the path forward. While some believe considerations surrounding the patient and device should be suspended until drug attributes are clearly defined, this approach can lead to decision making on device development or selection that can take a developer too far down a development path to make meaningful changes.
While usability testing activities are well understood when it comes to device selection and are critical at this stage, discovery research is a fundamentally different type of activity that seeks to define and understand user needs rather than help ensure they are met. User needs should be characterised as soon as there is a known target patient population – to most effectively drive the development of solutions covering the device, connectivity, mobile applications, data analytics and enhanced training that address the whole of the patient journey. The outputs from this effort include patient journey maps, clinical process maps, an understanding of prioritised user needs and values, and pain points that can be leveraged to improve the patient and provider experience (Figure 3).
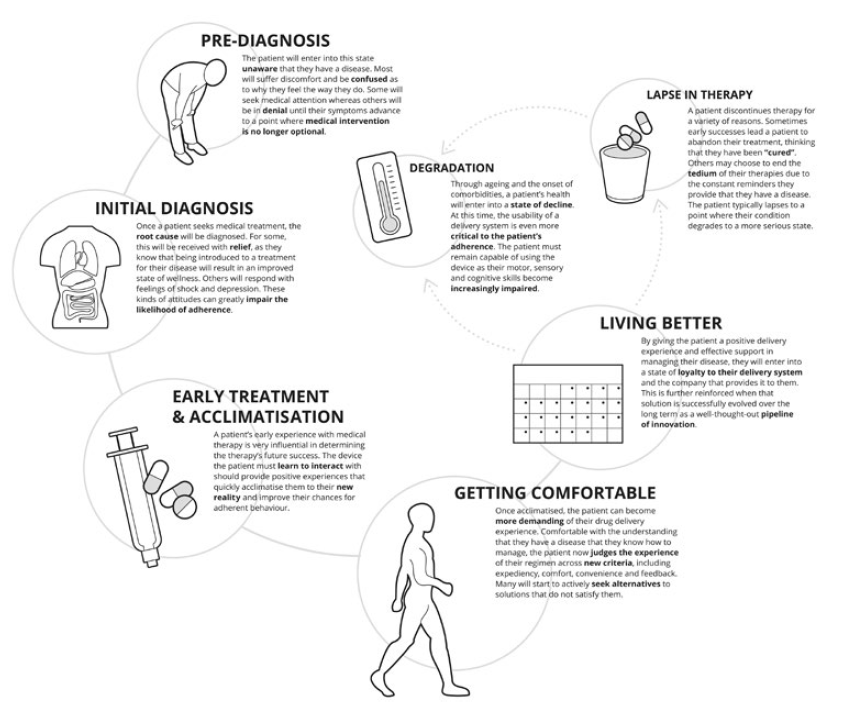
Figure 3: Patient journey.
“The focus has been, when designing the product, on an automated and safe injection process totally invisible for the user as well as secure administration of the prescribed drug.”
OPTIMISING THE USER EXPERIENCE
Ultimately, this information can be synthesised into a development framework. This consists of mapping identified needs with the corresponding device and supporting element opportunities and capabilities. The road map can then be used to guide early mapping of the envisioned ideal patient and healthcare professional journey, and the process of exploring solutions that best meet the needs at each phase of the journey while enabling the team to consider solutions more holistically.
This is particularly important when determining where the integration of connectivity is that can meaningfully support the experience. The road map also becomes the foundation for later requirements definitions for the device, the mobile application and any other clinical interface required, ensuring that requirements stem from user needs, rather than known technical capabilities and limitations. This helps developers ensure that all elements of a device strategy for a new drug product effectively address user needs optimally and holistically (Figure 4).
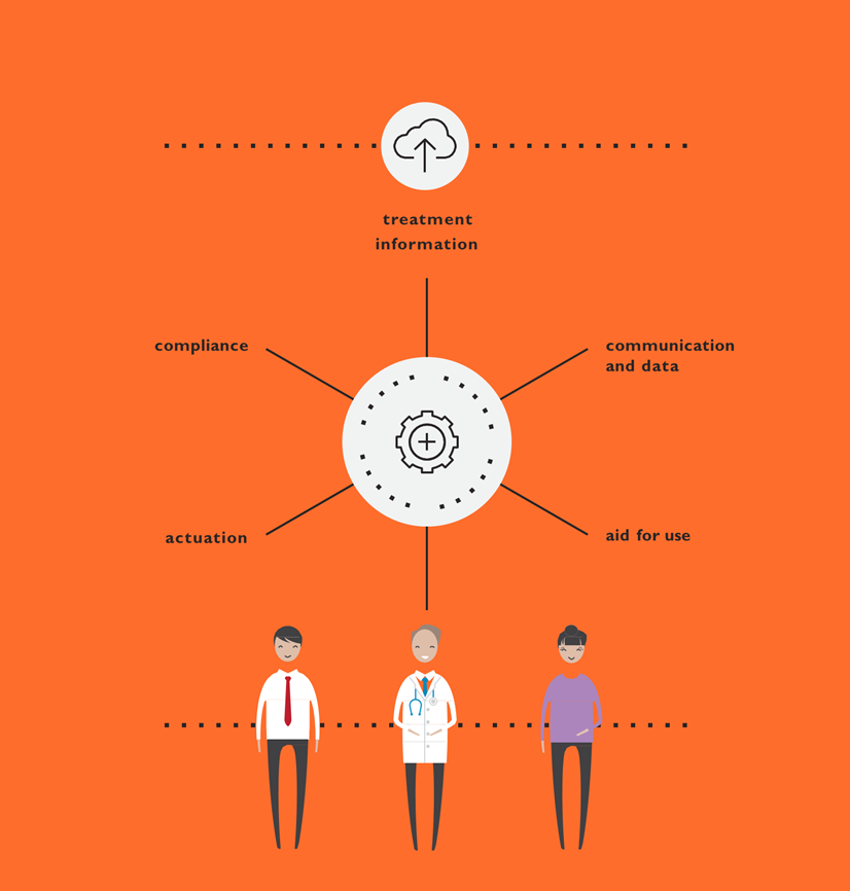
Figure 4: Connected devices.
Nemera’s Insight Innovation Center can use this foundation to develop all device aspects, from product design to mobile app user interface development, through an iterative process of design and evaluation sprints that integrate touch points with patients and clinical stakeholders to ensure solutions optimising all aspects of the experience and providing customers with robust assets for implementation by software development teams. Beyond mobile apps, needs can be addressed holistically in terms of packaging, both paper and digital instructions for use, and training. This approach of “surrounding” a device ensures that the entire experience is assembled by design and not in a piecemeal approach. Assets such as the mobile app for device connectivity can be used in clinical trials as well to monitor patient experience and provide critical feedback that can be incorporated into the system prior to commercial launch.
APPLICATION IN A REAL CASE: SYMBIOZE®, A SMART AND SUSTAINABLE ON-BODY INJECTOR
Targeting mid- to long-term medication in therapeutic areas such as oncology or immunology, the Symbioze® drug delivery system is a connected and reusable on-body injector suitable for large-volume injections.* The use of such devices is more than likely to expand, as many drugs in these fields are transferred from intravenous to subcutaneous delivery, which makes the disease burden even more cumbersome on the patients’ shoulders. To alleviate part of this burden, the focus has been, when designing the product, on an automated and safe injection process totally invisible for the user as well as secure administration of the prescribed drug. Therefore, combining electronics and “proximity card” technology is Nemera’s choice to address these issues. In the same way, a Bluetooth connectivity feature has been built in to allow functionality such as data recording and sharing, notifications and reminders.
Although such a feature is common and well accepted in diabetes care, it remains innovative in cancer treatment and inflammatory pathologies (Figure 5).

Figure 5: Symbioze®, smart & sustainable on-body injector platform to improve patients’ injection experience.
To refer to the example above, oncology patients need, on top of a seamless device, to achieve treatment adherence outside the hospital setting successfully, as well as to keep a link with remote healthcare professionals. Symbioze® can be easily integrated into the patient journey.
To fulfil these requirements, Nemera has chosen to entrust the German company Zollner (Zandt, Germany). This new alliance will enable Nemera to offer enhanced connected drug delivery device solutions to customers and patients. As a partner of choice, Zollner will support the design, development and manufacturing of electronic drug delivery systems for both Nemera’s proprietary and customer-owned products.
BENEFITS OF PARTNERING WITH AN INTEGRATED PRODUCT AND SERVICE PROVIDER
Successful navigation of these challenges often requires a partner with a broad set of capabilities, products and services. Nemera’s integrated development, consulting and manufacturing services guide its customers from understanding the patient journey to inform device design decisions, through to clinical trials, registration and, ultimately, to market introduction and beyond with a single partner applying an agile process across the device and combination product value chain. Nemera can support every device strategy a customer may be considering, from organic development to use of its intellectual property platforms, as well as a wide range of services to support the customer’s journey to a combination product. This is linked to Nemera’s global manufacturing footprint. Ultimately, the benefits of this approach will drive:
Patient-Centricity and Engagement
The needs of patients and clinical stakeholders are centred from the onset of the programme to develop a customised patient engagement strategy that is consistently considered throughout the process to maximise effectiveness when launched into the market to drive loyalty from stakeholders.
Innovation Across the Journey
Nemera’s world-class design, development and its Insight Innovation Center ensures the deployment of innovative development strategies as unique as its customers’ apps for both intellectual property products and custom development services regardless of point of engagement.
Reduction of Risk and Increased Speed of Market Access
A single partner working across the journey limits transitions between suppliers and ensures consistent execution of strategy. Nemera also provides a wealth of options for developing and realising a device to accelerate time to registration and market from small-series to large-scale manufacturing and interim supply required in between. When combined with Nemera’s service offering, this can significantly reduce timelines.
* Symbioze® is a trademark of Nemera la Verpillière SAS registered in the EU and pending in the US.
REFERENCE
- “SmartPilot – Go for Smart Guidance”. Company Web Page, Ypsomed. (https://yds.ypsomed.com/en/injection-systems/smart-devices/smartpilot-for-ypsomate.html, accessed November 15, 2022)

