To Issue 137
Citation: Gross C, “Offering a Smart and Sustainable On-Body Injector for Chronically Ill Patients”. ONdrugDelivery, Issue 137 (Sep 2022), pp 6–9.
Cécile Gross discusses the unmet drug delivery needs in chronic diseases, and details how Nemera’s answer – the Symbioze® on-body injector – fulfils these needs.
“Apart from the rapid and continuous rise in biologics, three other major trends play a prominent role in the drug delivery devices field – home administration, digital connectivity and environmental sustainability.”
When talking about chronic conditions and long-time treatments, diabetes frequently emerges as the first topic of conversation. This is unsurprising as it has been prevalent in medical discussions since the first insulin injection to a human being, which was administered a century ago. However, diabetes is not the only chronic disease that inflicts a cumbersome daily burden on patients’ lives. On the drug development side, much progress has been made, with new medicines enabling a broader range of pathologies to be addressed with biologics, while on the delivery side, technical innovations and patient awareness have led to an increased variety of well-accepted devices.
So what’s next? According to recent analysis,1 the two key therapy areas of focus are oncology and immunology, with a compound annual growth rate estimated at approximately 10% through 2026. Oncology will offer new treatments, whereas immunology will benefit from an increased number of treated patients and several new products, before the arrival of biosimilars. Another fast-developing area is neurology, especially with novel therapies being developed for migraine, Alzheimer’s disease and Parkinson’s disease.
Apart from the rapid and continuous rise in biologics, three other major trends play a prominent role in the drug delivery devices field – home administration, digital connectivity and environmental sustainability. As a device manufacturer, Nemera’s goal is to try to square this circle, integrating every constraint put forth both by its pharma partners and patients with chronic conditions.
ADDRESSING THE FULL RANGE OF UNMET NEEDS WITH ONE DEVICE
The idea to offer a smart, sustainable on-body injector to chronically ill patients stems from the unmet need to simultaneously address the challenges of biologics, self-administration, connectivity and sustainability in a single device. The need for high volume delivery and the limitations of current injectable drug delivery devices have already been stated in previous articles.2 Combined, these factors naturally lead to on-body injectors as the solution. They are ideal for the administration of biologics in general, and more specifically for the drugs used in therapeutic areas such as oncology, immunology, haematology, neurology and immuno-oncology.
Nemera has chosen its own distinct way to address the challenges of high-volume self-administration. First, by studying the state-of-the-art technology in the field, it became clear that such a device could benefit from miniaturised electronic components. Just taking a look at recently released insulin pumps can provide an insight into what device developers are capable of today. Additionally, reliable large-volume primary drug containers are now available as a standard offering, which makes both in-house integration and compliance to pharma’s fill-finish facilities easy.
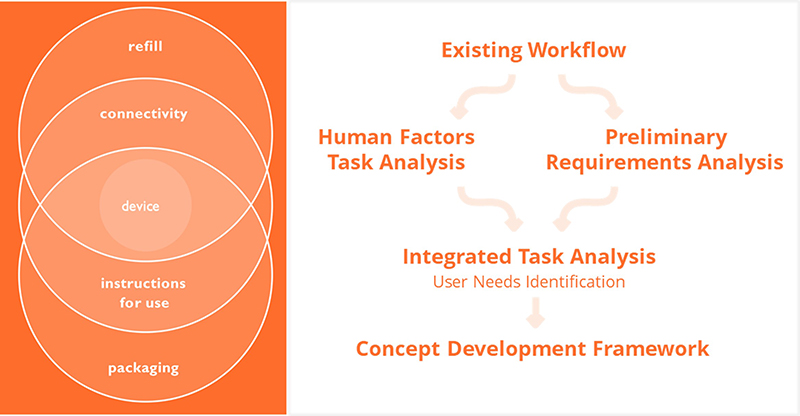
From a user perspective, an on-body injection device should be ideal for administering multiple drug profiles for multiple uses, so integrating user needs from the start of development is crucial. Nemera has leveraged the expertise of its Insight Innovation Center3 to map out the patient journey and set out a detailed human factors programme for the different phases of development, including the patient experience from healthcare professionals.
Referring to immuno-oncology, where ease of administration and cost of therapy have been identified as primary unmet needs, Nemera’s final device design offers a user-friendly operating protocol and a combination of reusable and disposable modules – a perfect harmony for a device named Symbioze® (Figure 1).

Figure 1: Symbioze® – a smart and sustainable on-body injector platform to improve patients’ injection experience.
“Offering a high level of ease-of-use facilitates prescription and training from healthcare professionals. The result is a “Click, Patch and GO” device.”
SYMBIOZE®: THE ADDED-VALUE OF A PLATFORM APPROACH
Needless to say, implementing a platform strategy for a high-volume on-body injector has enabled Nemera to leverage baseline knowledge and generated data throughoutthe development process. This platform can be tailored to a specific combination product to meet pharma customers’ needs.
To be more precise, this added value can be detailed into:
- Broad device versatility
- Delivery and device customisation
- High compatibility with different primary drug container manufacturers
- Controlled development lead-time
- Acknowledged cost-effectiveness.
The main characteristic of the device is the split between a reusable module and a disposable one (Figure 2). The disposable module is totally linked with the drug and its injection. As such, all the features ensure proper, complete and safe administration of the dose.
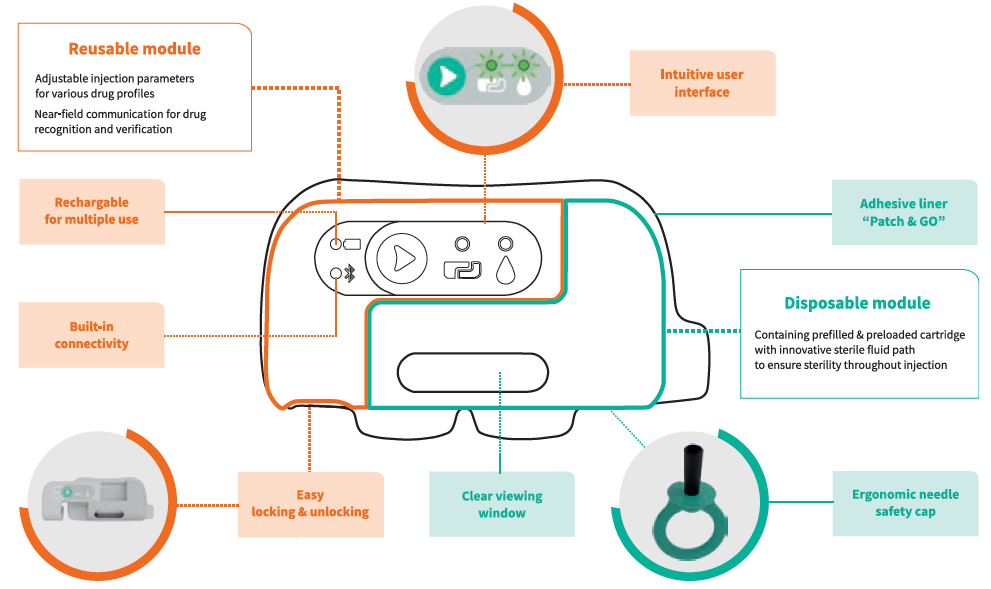
Figure 2: Symbioze® comprises a reusable and a disposable module, offering key benefits to administer complex drugs.
Symbioze®’s near field communication connectivity feature provides reassurance for both users and pharma companies regarding the administration of the prescribed drug. The transparent window enables a clear visibility of the drug and its container prior to the injection, of the injection progress during the injection and of injection completion once the full dose is administered.
There is no need to load the primary drug container nor to adjust any parameter as the module comes pre-loaded and pre-set. The ergonomic needle shield is highly visible to indicate to the user where to pull the cap from the module. It is also hollow and round to increase the ease of its withdrawal. Removal of the needle shield does not make the needle apparent or increase the risk of needlestick injury, as the insertion and retraction of the needle are automated once the start button is activated.

Figure 3: Simulated use of Symbioze® on-body injector prototype.
Last but not least, the adhesive liner is ready to use when the user has the module in hand and, given its shape, the correct way to place the device is naturally obvious. The process could be simply summed up as “Patch and GO” (Figure 3).
The reusable module contains the electro-mechanical part, which needs to be charged. The battery LED indicates device autonomy and the USB connector allows recharging. This connector is protected, as it is placed inside the module, but it stays easily accessible. There is another LED indicator for the Bluetooth connectivity. This connectivity can be used in features that help fight against the decreasing patient adherence rate over the months.
The user interface has been improved as a result of insights gained during the first user study performed in-house and is now restricted to a single green button. Once it is activated, the device “wakes up” and the LED that indicates the connection of the two modules lights up. The second LED indicator is related to the injection readiness.
The tricky challenges of the device using two separated modules (one containing the drug and the other containing the necessary electronics for the injection) are related to the integrity of the drug, which is key to achieving the expected clinical outcomes, as well as ensuring the sterility of the primary drug container in a non-sterile environment. Nemera’s R&D teams have addressed these challenges successfully and provided an innovative solution that does not compromise the user-friendliness of the device.
Benefitting from Nemera’s experience in autoinjectors, special attention has been paid to the number of user steps; be it for a patient or a caregiver, a treatment-naïve or an experienced patient. In the same way, offering a high level of ease-of-use facilitates prescription and training from healthcare professionals. The result is a “Click, Patch and GO” device. Once the injection is complete, the two modules can be separated, and the disposable module discarded in a sharps container or biological waste bin.
To follow this user focus and act on Nemera’s motto of “patients first” (Figure 4), several studies have already taken place and complementary ones will be performed as the product matures. The latest one was conducted at the company’s Insight Chicago location and involved both healthcare professionals and chronically ill patients. The patients were recruited in the following categories:
- Oncology (e.g. multiple myeloma, acute myeloid leukaemia, lung cancer, breast cancer)
- Autoimmune diseases (e.g. rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis, atopic dermatitis, psoriasis)
- Haematological immune deficiencies (e.g. Haemophilia A)
- Disorders of the central nervous system (e.g. multiple sclerosis, Parkinson’s disease, Alzheimer’s disease).

Figure 4: Supporting the combination product ecosystem from the earliest stages of developing a device strategy.
The study was structured in four phases, starting with an introduction to the device, going on to “regular” simulated use, then a deviation from regular simulated use and a post-study interview. The general feedback was good and improvement activities have already started.
Within a continually evolving environment, offering simple care in complex devices is a demanding but exciting challenge. Being part of the adventure with an innovative smart and sustainable on-body injector for chronically ill patients is a key milestone for Nemera. All the company’s teams are ready to accompany its pharma partners in the journey to achieve a patient-centric combination product.
REFERENCES
- “The Global Use of Medicines 2022 – Outlook to 2026”. IQVIA presentation, Jan 2022.
- Duband S, “Tackling High-Volume Administration Challenges with a Smart, Sustainable On-Body Injector Platform”. ONdrugDelivery, Issue 124 (Sep 2021), pp 36–40.
- Tunkel M, Sapatnekar N, “Holistic Development and Industrialisation of Drug Delivery Devices and Combination Products”. ONdrugDelivery, Issue 136 (Aug 2022), pp 39–42.