To Issue 145
Citation: Suman J, Karavas N, “The Potential and the Challenges of Nasal Vaccination”. ONdrugDelivery, Issue 145 (Apr 2023), pp 66–70.
Julie Suman and Nektaria Karavas discuss the challenges and opportunities of intranasal vaccination – showcasing current understanding for both liquid and powder products. Experimental studies highlight critical issues, the performance of commercially available devices and developments in formulation practice.
Nasal vaccines are intuitively logical for pathogens that enter via the nose, so it is unsurprising that the SARS-CoV-2 pandemic has reinvigorated interest in intranasal vaccine development. Globally there is growing recognition that such products could support efficient long-term management of the SARS-CoV-2 virus, as evidenced by remarks from the White House Summit in the US on next-generation vaccines.1
“Mucosal immunisation has the potential to alter disease trajectory by tackling the virus earlier, slowing any advance from the nasal cavity.”
Four intranasal vaccines have already been approved for use,2 most recently iNCOVACC (Bharat Biotech, Hyderabad, India) and Convidecia Air™ (CanSino Biologics, Tianjin, China) – and many more are in development.1 More generally, the attractions of intranasal vaccination point to potential for other indications, such as respiratory syncytial virus.
Developing nasal vaccines is complex but the associated knowledge base is advancing rapidly, led by companies such as Aptar Pharma, which offers cutting-edge solutions and services to support commercialisation – incorporating an expanded skillset in nasal formulation by Nanopharm, an Aptar Pharma company.
WHY VACCINATE INTRANASALLY?
Intranasal vaccination offers the potential of mucosal immunity. The SARS-CoV-2 virus enters the body via the nasal and oral passages, subsequently replicating, shedding and translocating to other areas, notably the lungs. Mucosal immunisation has the potential to alter this disease trajectory by tackling the virus earlier, slowing any advance from the nasal cavity. In addition, mucosal immunity may prevent disease spread by limiting viral shedding. By enabling mucosal plus systemic immunity, alone or in combination with established intramuscular vaccines, intranasal vaccination offers better protection against SARS-CoV-2, along with improved patient outcomes.
Clearly, this clinical advantage is critical, not just for SARS-CoV-2 but also for other respiratory viruses that share its method of attack. But intranasal vaccination also comes with additional practical advantages, including:
- Elimination of needles, improving safety for healthcare practitioners and encouraging uptake by needle-phobic patients
- Easy delivery, including potential for self-administration
- Reduced waste by eliminating use of syringes, bandages and alcohol swabs
- Opportunities to minimise cold-chain requirements with powder-based formulations.
DEFINING REQUIREMENTS FOR THE DELIVERY OF NASAL VACCINES
Figure 1 highlights the nasal-pharyngeal-associated lymphoid tissue (NALT) that constitutes the nasal immune system and is consequently the primary target for intranasal vaccination. Dendritic cells in the nasal epithelia provide a secondary route for vaccine entry. Current research also indicates the importance of targeting the nasopharynx and throat.
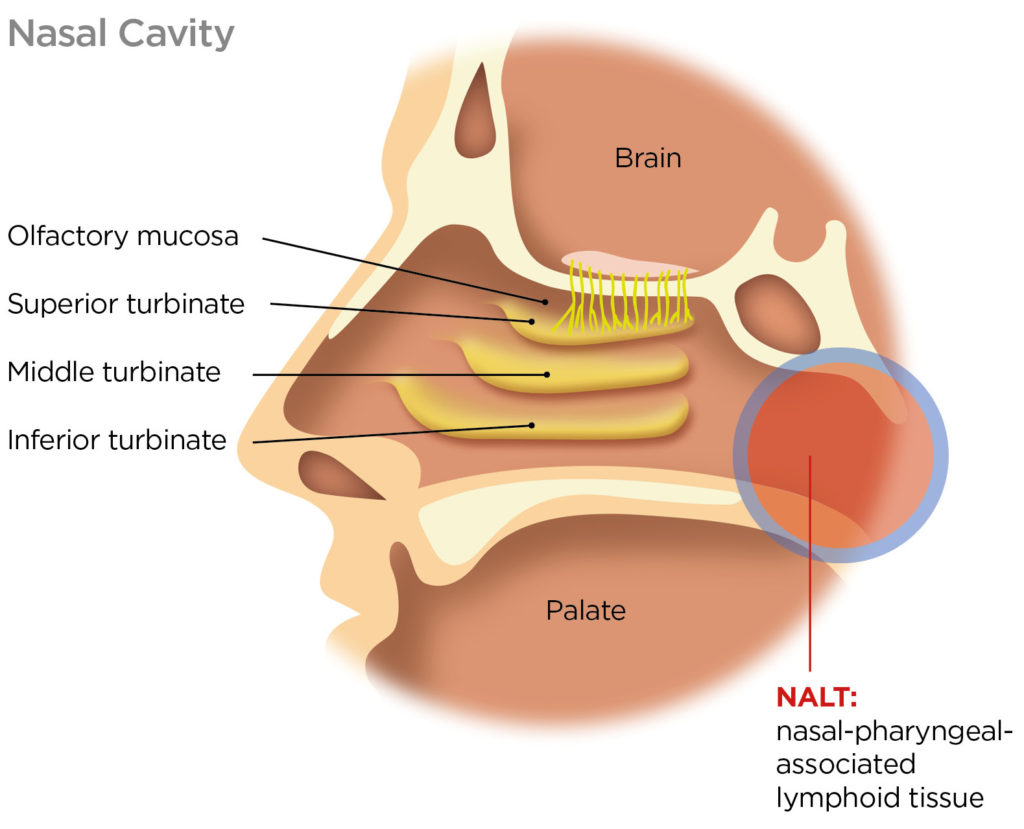
Figure 1: Side view of the nasal cavity highlighting the NALT, the primary target for intranasal vaccination.
“Formulators must safeguard the stability of the antigen, preventing aggregation and physical and/or chemical degradation, and maximise immunogenicity.”
To make an effective nasal vaccine, the device and formulation parameters need to be manipulated to transport the antigen efficiently to these target sites. The need for effective targeting is exacerbated by the risk of nose-to-brain drug delivery, which is reduced by minimising deposition in the olfactory region/superior turbinate. Particle size is a defining parameter with respect to in vivo deposition, with a delivered droplet or particle size in the region of 20–40 μm typically optimal. Particles in the sub-10 μm size range are prone to pulmonary delivery – triggering additional safety/toxicity concerns.
Other aspects of the physiology of the nasal cavity also require careful consideration. Firstly, it is a mildly acidic environment. Formulations that mirror this acidity and are also isotonic are therefore better tolerated. Positively charged particles interact more effectively with cell membranes and are associated with a better immune response. Enzymatic activity is limited compared with the gastrointestinal tract, but mucociliary clearance is a defining concern.
Mucociliary clearance is highly effective in sweeping contaminants towards the back of the nasal cavity, typically renewing the mucus layer every 15–20 minutes in a healthy adult. To navigate it, antigens must diffuse rapidly from the formulation into target cells. This is a major challenge, a clear differentiator relative to formulation for intramuscular delivery and a complicating factor for a direct switching of delivery routes.
Developing intranasal vaccines to meet these outlined requirements involves the twin-track optimisation of device and formulation – the tuning of a formulation for optimal performance in a specific commercially viable device, taking into account patient technique. Aptar Pharma supports customers through this complex optimisation process with an established portfolio of device platforms and exemplary formulation skills, thanks to the knowledge base at Nanopharm.
FORMULATING FOR INTRANASAL VACCINATION, LIQUID OR POWDER
Aqueous formulations for intranasal vaccination are often relatively simple. Formulators have comparatively few approved ingredients to work with, creating a need for skilful optimisation with the tools available. Some or all of the following ingredients are commonplace:
- Adjuvants
- Buffers
- Amino acids
- Sugars
- Viscosity modifiers
- Osmolality modifiers.
“The availability of appropriate platforms for clinical and commercial use is a vital enabler for intranasal vaccine development.”
Using these components, formulators must safeguard the stability of the antigen, preventing aggregation and physical and/or chemical degradation, and maximise immunogenicity. Adjuvants promote a stronger immune response although choice is limited to, for example, certain bacteria, cytokines, nucleic acids and virus-like particles. Within stability constraints, pH and osmolality can be optimised for comfort/tolerance. Viscosity modification is an important lever for efficient dispersion with the device of choice. Increasing viscosity may also improve retention in the nasal cavity and slow mucociliary clearance.
Dose volume is an important consideration for aqueous nasal vaccines, with considerable variation observed in commercial products. For example, influenza vaccine FluMist (MedImmune, MD, US) involves the delivery of a single 200 μL spray shot, while the total dose volume for iNCOVACC is 500 μL delivered as a series of nasal drops. Optimising dose volumes for specific delivery platforms remains a work in progress.
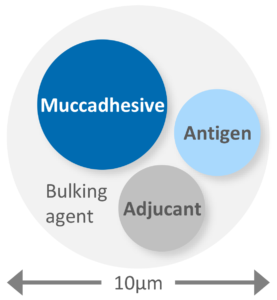
Figure 2: Powder formulation calls for the engineering of particles larger than 10 μm in which the required constituents are uniformly distributed in a form that promotes rapid and effective activity upon deposition.
Formulation as a powder is appealing as it addresses certain challenges associated with aqueous nasal vaccines. Vaccine powders negate any requirement for cold-chain storage, enable the delivery of higher payloads and offer inherently enhanced stability for biologics. Just like their aqueous counterparts, powder formulations include the antigen and adjuvant (if used), but often other uniquely essential components too, such as mucoadhesive agents, bulking agents and matrix stabilisers (Figure 2).
Preparation method is an important consideration for powder formulations since sophisticated particle engineering is required to ensure the uniform distribution of all the required components in a form that promotes rapid release and activity, post-deposition. As with aqueous formulations, the target delivered size range is >10 μm, although the optimal size for antigen uptake by dendritic cells is in the region of just 200–300 nm;3 nanostructured vaccine vehicles are an important area of development. Lyophilisation or freeze drying is the conventional preparation technique but alternatives such as thin film freeze drying, spray drying and/or vacuum drying are all promising with respect to producing stable dispersible particles with desirable properties for product manufacture.
DEVICE DESIGN: SURVEYING THE OPTIONS
When it comes to devices, the availability of appropriate platforms for clinical and commercial use is a vital enabler for intranasal vaccine development, for both aqueous and powder formulations. Key considerations include:
- Dispersion/deposition characteristics – is the device effective in dispersing particles or liquid to the required size?
- Ease of use – will healthcare professionals be able to deliver the vaccine efficiently with minimal training?
- Patient independent performance – will every patient receive the required dose, regardless of technique? Even with self-administration?
- Availability – is the device suitable for clinical trials? Can it be scaled rapidly for commercial delivery?
- Compatibility with existing vaccine platforms – can the device be used with existing vaccine bottles and vials in formulation manufacture, storage and delivery?
- Price – will the overall vaccine solution be commercially viable?
These considerations guide the ongoing evolution of Aptar Pharma’s portfolio of vaccine delivery platforms (Figure 3). The company’s range includes options for both aqueous and powder formulation, prefilled/ready to use or transfer, single or multidose. BiVax and LuerVax are suitable for use from clinical trials to commercial use, while CPS, Unidose and Bidose already fulfil market requirements for off-the-shelf solutions for commercial use.
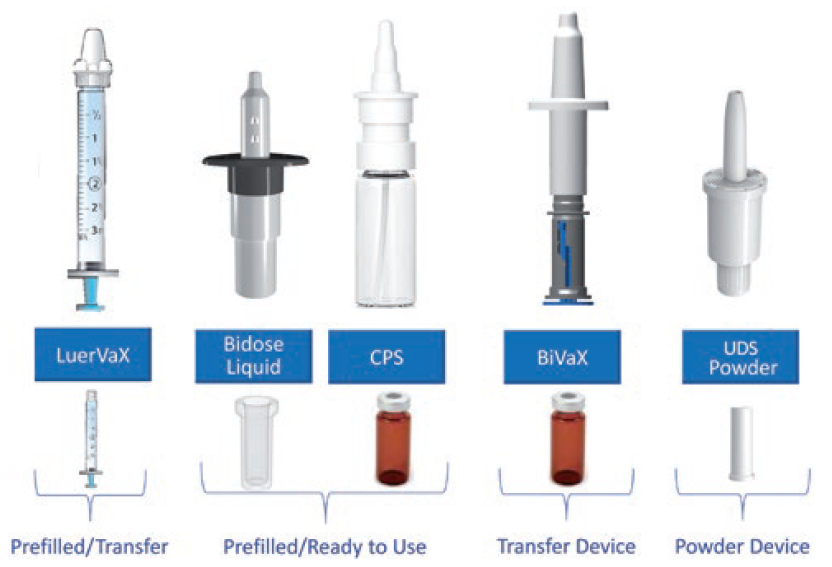
Figure 3: Aptar Pharma offers a comprehensive range of devices for intranasal vaccination, antivirals and immunostimulants that facilitate efficient development through to commercialisation.
BiVaX is supplied as a complete assembled transfer device for aqueous or reconstituted formulations. Suitable for use with established vaccine containers – standard vials – it comes in two variants, 200 or 500 μL. An integrated dose divider simplifies dosing to each nostril.
LuerVax is a nozzle-based device for use with any Luer lock syringe. It can be used to develop either a prefilled-syringe-based solution or for vaccine transfer at the point of need. A dose divider enables the sequential delivery of two equal doses.
CPS is a multidose spray delivery platform for dose volumes from 50 to 140 μL. Interfacing directly with multidose vials, for easy product manufacturability, it is suitable for preservative-free formulation and can be irradiated, if necessary.
Unidose Powder and Bidose Liquid are convenient, prefilled, primeless single-use devices for the delivery of a single powder dose (10–80 mg) or two aqueous doses (200 μL in total), respectively. Offering easy, one-handed actuation and patient independent performance, they are manufactured at commercial scale for marketed products.
The following case studies highlight aspects of performance of some of these devices and exemplify the work Aptar Pharma does to help customers use them to successfully develop intranasal vaccines.
“Intranasal vaccination has broad potential for both prophylactic and therapeutic applications.”
Developing Nasal Vaccines: Exploring the Impact of Delivery Technique
Figure 4 shows data from an investigation of the impact of the holding angle, the angle of the device relative to the horizontal, and insertion depth on the deposition performance of BiVax. The graphs show data for holding angles of 30°, 45° and 50° at an insertion depth of 18 mm. Deposition data were measured using a nasal cast (Aeronose) for the nose, turbinate and nasopharynx regions.
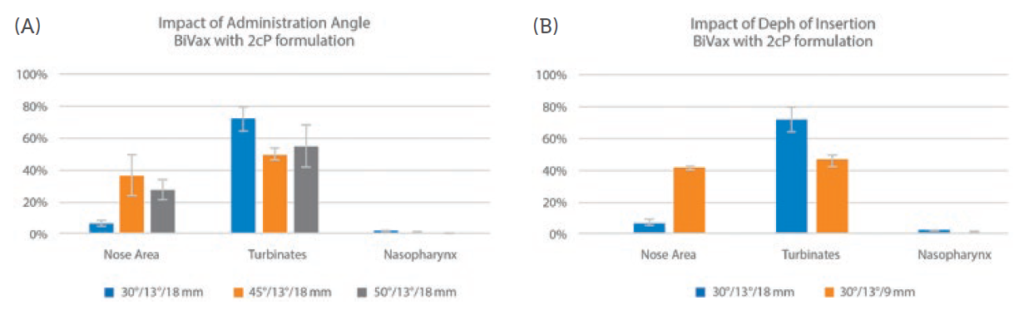
Figure 4: A shallower holding angle and shorter insertion depth favour deposition in the turbinates, suggesting better performance for vaccine delivery.
The results show minimal deposition in the nasopharynx, where the NALT would be expected to lie, but relatively high levels of deposition in the turbinates. The potential for mucociliary clearance to sweep formulation from the turbinates to the NALT make these data significant for vaccine delivery. Both holding angle and insertion depth affect deposition profiles, with a shallower holding angle and shorter insertion depth favouring higher deposition in the preferred area.
Developing Nasal Vaccines: Considerations for Nucleic-Acid-Based Formulations
While nasal vaccines may use different platforms, including nucleic acids such as mRNA and DNA, such nucleic acids are prone to degradation – necessitating the use of novel formulation strategies for successful product development. In this example, Nanopharm collaboration with the University of Bradford (UK), successfully developed and characterised a liposomal suspension loaded with a model small nucleic acid – herring sperm DNA – for nasal delivery.4 A nasal formulation matrix was made using trimethyl chitosan (TMC) as a mucoadhesive agent with adjuvant properties. The resulting formulations, with empty and DNA loaded liposomes, were put into an Aptar preservative-free CPS pump, 100 μl for in vitro performance evaluation.
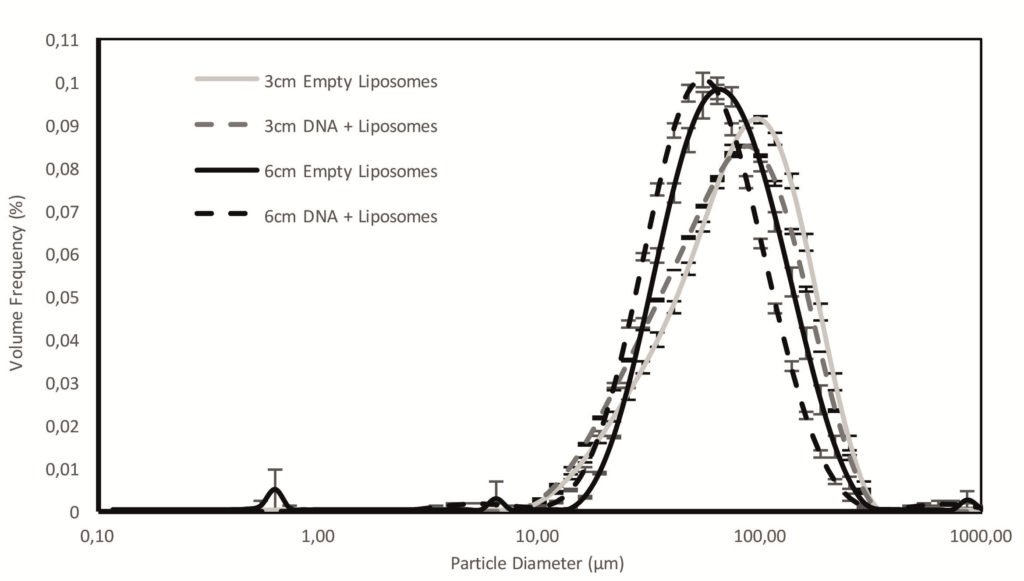
Figure 5: DSD data for empty and DNA-loaded liposomes formulations sprayed with Aptar CPS pump measured at 3 and 6 cm from the nozzle illustrate dispersion to a suitable size for nasal drug delivery (average and bars represent standard deviation, n = 3).
Both empty and DNA-loaded liposomes were successfully manufactured and formulated for nasal delivery, with the pH and osmolality of the resulting formulations compatible with the nose. The droplet size distribution (DSD) of the emitted sprays (Figure 5) was in the range for nasal deposition, although towards the upper limit. Reducing the amount of TMC could help in producing smaller droplets, thereby potentially increasing deposition at the target region (NALT and turbinates).
Table 1 shows DLS data measured for the formulations before and after spraying. These confirm that liposomal structure and size is successfully preserved through the spraying process.
| Formulation | Polydispersity Index |
Hydrodynamic Diameter (nm) | Zeta Potential (mV) |
| Empty liposomes | 0.24 ± 0.02 | 404.80 ± 11.59 | + 37.40 ± 1.13 |
| Empty liposomes after spraying |
0.22 ± 0.01 | 432.33 ± 3.59 | + 34.23 ± 0.98 |
| DNA-loaded liposomes | 0.20 ± 0.03 | 661.33 ± 27.14 | + 36.43 ± 0.97 |
| DNA-loaded liposomes after spraying | 0.13 ± 0.03 | 586.93 ± 11.05 | + 34.40 ± 1.07 |
Table 1: DLS measurements for empty liposomes formulation and DNA-loaded liposomes formulation before and after spraying show that structure is successfully preserved through the spraying process (average ± standard deviation, n = 3).
CONCLUSION
Drawn into the spotlight by the SARS-CoV- 2 pandemic, intranasal vaccination has broad potential for both prophylactic and therapeutic applications. Realising this potential is high on the priority list for many pharmaceutical companies and Aptar Pharma has built a service offering to help. From cutting-edge formulation expertise from Nanopharm and an unrivalled portfolio of ready-to-go device platforms, through regulatory filing to patient training and onboarding tools, the company’s integrated family of solutions can help clients progress faster to a better intranasal vaccine solution (Figure 6).
Figure 6: Aptar Pharma service offerings.
For more information about nasal vaccination, visit: https://www.aptar.com/products/pharmaceutical/nasal-vaccines
REFERENCES
- Buntz B, “White House holds nextgen COVID-19 vaccine summit”. Drug Discov Dev, Jul 26, 2022.
- Waltz E, “China and India approve nasal COVID vaccines – are they a game changer?”. Nature, Sep 7, 2022.
- Hellfritzsch M, Scherliess R, “Mucosal Vaccination via the Respiratory Tract”. Pharmaceutics, 2019, Vol 11(8), p 375.
- Blanes CR, Beaird R, “Formulation and characterisation of liposomes loaded with a model small nucleic acid for nasal delivery”. Drug Delivery to the Lungs, 2022, Vol 33.

