To Issue 140
Citation: Gandhi R, Budd G, “Steps to Success in Nose-to-Brain Drug Delivery”. ONdrugDelivery, Issue 140 (Nov 2022), pp 9–14.
Reenal Gandhi and Gemma Budd consider the multiple hurdles associated with developing nasal drug delivery solutions, current understanding of the associated biology and the advantages of alternative device designs and state-of-the-art formulation practice.
More sophisticated product development strategies, new biologic drug molecules and drug reformulation are all defining features of the current nasal drug delivery landscape. The development of an intranasal (IN) form of naloxone, a drug traditionally delivered by injection for the emergency treatment of opioid overdose, provides a good illustration of the benefits of reformulation, and of IN drug delivery more generally. Easier and quicker to use in a crisis scenario, by a broader range of responders, this product delivers the rapid onset required to save lives; estimates indicate a global market value in excess of US$350 million (£309 million) (2021 figures).1
“Aptar Pharma has been leading the way in nasal drug delivery solutions for decades.”
Drug reformulation has similarly delivered successful – and, in some cases, breakthrough – therapeutics for the treatment of seizures, hypoglycaemia, dry eye disease, treatment resistant depression (TRD) and migraine. But there are even bigger targets on the horizon. More effective solutions for anaphylaxis, for example, and nasal vaccines, including for covid-19, would similarly benefit from IN formats due to high patient acceptability and ease of use, alongside equivalent or enhanced therapeutic efficacy. The exponential growth of research demonstrating the viability of nose-to-brain drug delivery holds promise for efficacious treatments for disorders of the central nervous system (CNS), which would be life transforming for many.
Products commercialised in nasal dosage forms for conditions primarily of the CNS, such as TRD, have been approved based on their systemic absorption, but there is growing evidence to suggest that their improved efficacy (as compared with traditional dosage forms) might result from some drug delivery directly into the brain. Pharmaceutical companies and researchers are expending significant efforts to understand and facilitate this pathway to address previously unmet medical needs for diseases of growing incidence, such as Alzheimer’s, Parkinson’s and depression.
Individually, nasal drug delivery and drug delivery to the brain both present specific challenges. Add the two together and you have an exacting and demanding task, with much of the associated knowledge base still evolving. Aptar Pharma has been leading the way in nasal drug delivery solutions for decades. Today, the company combines the widest range of proven nasal spray devices with a track record of success in supporting the commercialisation of nasal drug products targeting the CNS.
“The nasal cavity offers a highly vascularised space for rapid absorption into the blood.”
THE NOSE-TO-BRAIN DRUG DELIVERY OPPORTUNITY
Conditions such as allergic rhinitis were the earliest targets for nasal drug delivery and, in terms of volume, these topical therapeutics still dominate the market. However, there are a growing number of products that use the unique physiology of the nose to tackle very different disorders. The nasal cavity offers a highly vascularised space for rapid absorption into the blood, for systemic action. But, in addition, there is a direct pathway to the cerebrospinal fluid and brain tissues – a pathway that bypasses the blood-brain barrier (BBB).
Delivering drugs to the CNS via systemic delivery is extremely challenging. Metabolism, elimination and off-target delivery all limit efficiency, with the BBB presenting a formidable final hurdle, since transport across the BBB is largely limited to very low levels of very small lipophilic molecules. By bypassing the BBB, nasal drug delivery can result in a higher bioavailability of the drug in the brain, whilst administering lower doses and, at the same time, broadening the range of potential drug candidates. This increases the chances of achieving therapeutic efficacy with less toxicity and fewer side effects which, of course, is a major gain. The lack of any regulatory requirement for sterility is a potential additional benefit from a manufacturing perspective but can increase the complexity of formulation.
The Biology: How do Drugs Get From the Nose to the CNS and What are the Barriers?
Our understanding of how drugs travel from the nose to the brain is still advancing, but the current view is that extra or paracellular (between cell) transport, along the ensheathing channels of major nerves, is the dominant and fastest route. There is some evidence of intracellular (within cell) transport, but this appears to be much slower – a secondary mechanism that may enable the delivery of sustained therapeutic effect.
The nasal cavity offers access to two main nerves – the olfactory nerve and the trigeminal nerve (Figure 1). The olfactory nerve lies predominantly in the olfactory region – a small area deep in the roof of the nasal cavity that accounts for just 7% of the surface area of the nasal epithelia. Since the olfactory nerve is directly exposed to the local environment in this region, the primary challenge with this route is to reach and precisely target such a small area, preferably with a highly concentrated formulation to avoid spreading and off-target deposition. Penetration through the mucus is also critical to reach the interface with the neurons.
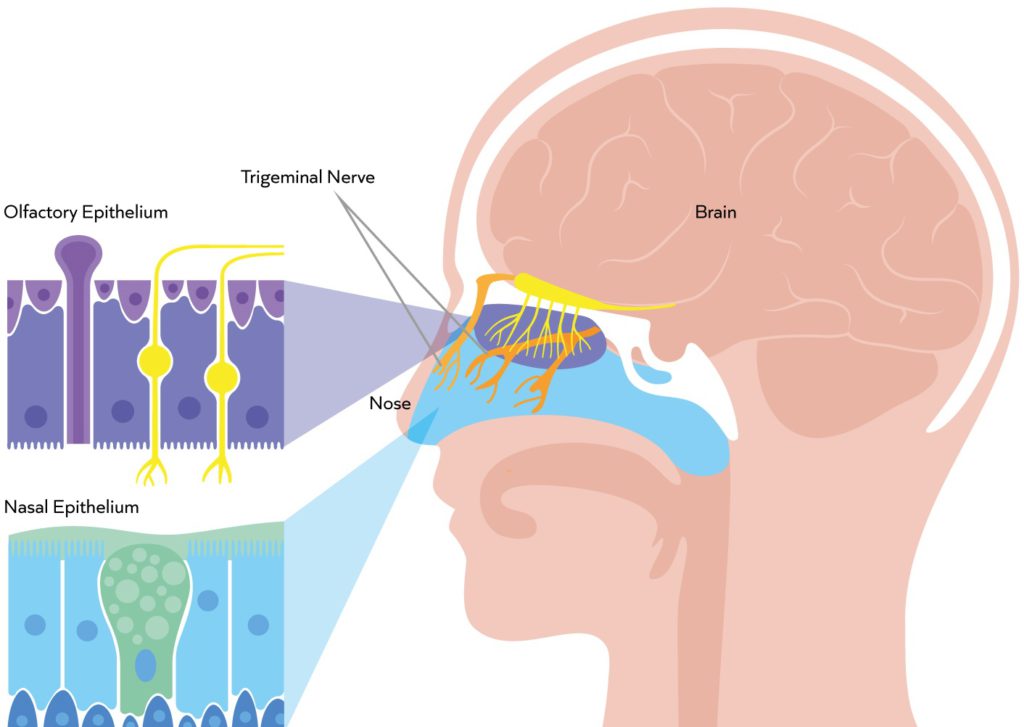
Figure 1: The exposed olfactory nerve in the olfactory region is a primary target for nose-to-brain drug delivery. The trigeminal nerve, within the turbinates, presents a larger surface area but lies behind tight cell junctions with access further compromised by mucociliary clearance.2
“Selecting a suitable nasal spray device is a critical aspect of product development.”
In contrast, the trigeminal nerve presents a much larger target surface area, extending through the turbinate region, which represents the majority of the nasal cavity. Since this area is subject to mucociliary clearance and the trigeminal nerve is hidden behind tight cell junctions, the defining challenge with this route is to overcome these barriers without simultaneously enhancing cellular and/or systemic uptake.
Alongside the specific challenges associated with each route, there are certain issues to address. The nasal cavity is a constrained space with a complex geometry and a narrow airflow path that contains little fluid. Reliably delivering a dose to a target site is therefore difficult, and drug dissolution (in the case of a powder) can be slow. The nose has evolved to prevent the permeation of unwanted molecules, with multiple natural defences – including the mucus barrier itself – alongside the presence of proteases and macrophages that will attempt to break down or scavenge foreign matter if countermeasures are not designed into the formulation.
The Nasal Spray Device: What Factors Affect Performance and Choice?
Selecting a suitable nasal spray device is a critical aspect of product development but it is important to recognise that it is a combination of the device, formulation properties and patient technique that determines deposition behaviour. Device designs can be optimised to produce different plume geometry, angle, velocity and droplet/particle size for a given formulation to maximise deposition in the upper nasal cavity/olfactory region. With respect to patient or caregiver technique, device administration angle and spray nozzle insertion depth can both influence deposition behaviour and design features can help here too – nasal guides being a prime example. Encouraging correct device position can reduce patient influence, promoting more reliable and consistent dose delivery.
Progressing device design relies on having effective tools for assessing performance. Translational models for nose-to-brain delivery are limited, primarily due to relative differences in the size of the olfactory region between humans and animal models. Optimising the relevance and application of in vitro tools is therefore crucial. Aptar Pharma has developed and clinically validated an in vitro nasal cast model, AERONOSE® Nasal Cast (co-developed and co-owned by Aptar Pharma, Diffusion Technique Française and the University of Tours, France) to support its device and product development activities. The cast makes it quicker and easier to understand the impact of different factors on product performance and is helping to optimise device design for deposition in the olfactory region.
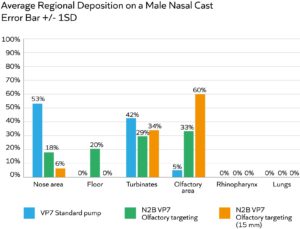
Figure 2: A multidose pump, optimised for olfactory targeting, substantially enhances drug deposition in this region relative to a standard multidose pump; increasing insertion depth to 15 mm (relative to 10 mm) is also highly beneficial.
Figure 2 shows data from an experiment to compare the olfactory deposition performance of two configurations of the same multidose liquid pump – the standard version (blue bar) and one with a modified actuator specified for olfactory targeting (green bar). The effect of device insertion depth was also assessed with the modified actuator device (orange bar). All trials were carried out using a low-viscosity placebo (2 cP) and an administration angle of 45°.
Here we can see that modifying the actuator and inserting the spray nozzle further into the nostril – to a depth of 15 mm relative to 10 mm – are both individually and cumulatively beneficial for olfactory deposition. These results underline the importance of device design and the value of nasal guides, which can be used to promote insertion to the optimal depth.
Via studies such as these, Aptar Pharma is progressively refining device designs for targeted delivery. To date, it has reached up to 60% deposition to the olfactory region with liquids (as shown in Figure 2) and 65% deposition with powder formulations – a major advance relative to unoptimised delivery devices. Commercial prototypes with design features such as nasal guides are already undergoing trials in the clinic.
The Formulation: How Can We Optimise Deposition, Retention and Permeation to the Target Site for Different Molecules?
The starting point for formulation is detailed characterisation of the physicochemical properties of the drug molecule and identification of the mode of action. Drug molecules vary considerably with respect to their fundamental properties and need different types of “help” to reach a target site of action. For example, some are hydrophilic, others are lipophilic – polar opposites. Differences relating to nasal delivery include cell and tight junction permeability, sensitivity to enzymatic degradation (notably for biologics), sensitivity to pH, and mucosal interaction and penetration. With an understanding of the inherent properties of the drug, formulation development can proceed via a process of knowledge-led pre formulation studies, typically followed by a design of experiments, because this is a complex process with multiple issues to address. The following, far from exhaustive, list gives a flavour of the issues that should be considered to reach an optimal solution:
Preservatives or Preservative Free
Non-sterile manufacture is an option for nasal sprays but preservatives are then required to deliver appropriate microbial integrity and an acceptable shelf life with a standard device. Where preservatives are used, compatibility with the drug molecule is a concern (particularly for biologics) and physiological effects, helpful or otherwise, require careful assessment. If sterile manufacture is a viable option (for example, if reformulating an injectable product, the sterile manufacturing process will already be in place), then maintaining sterility remains a challenge. Devices such as Aptar Pharma’s CPS spray pump platform, which has unique features preventing microbial ingress despite multiple actuations, are essential for successful preservative-free formulation.
Osmolality and pH
In an aqueous nasal spray, incorporating excipients or selecting buffer systems to promote drug stability and delivery may impact both the osmolality (ionic concentration) and pH of the formulation. However, total flexibility in this regard, is constrained by the biology of the nose, since specific ranges of osmolality and pH are needed to avoid mucosal irritation.
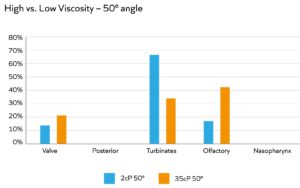
Figure 3: Higher viscosity is associated with an increase in deposition in the olfactory region in back-to-back studies.
Rheology and Thixotropy
Formulation rheology, including viscosity, has multiple impacts. Increasing viscosity tends to improve retention time, either by facilitating adherence to the mucus or hindering the ability of the beating cilia to clear away the drug substance. Higher viscosity can also facilitate better droplet-size control for more effective targeting to the back of the nose and olfactory region (Figure 3) and improve formulation stability, even at high drug loadings. However, device choice can be more limited for viscous formulations, and the emitted dose may emerge as a jet rather than fine droplets – giving rise to patient discomfort. A thixotropic formulation with shear-thinning behaviour can be the answer. Such formulations exhibit low viscosity when subject to high shear (i.e. when passing through the nasal-spray pump) but build higher viscosity upon settling, following deposition.
Formulation rheology is routinely manipulated using modifiers that often simultaneously and usefully exhibit muco-adhesive and/or mucopenetrative properties because of relationships between viscosity and cilia transport. The drug substance can also have an intrinsic effect on formulation rheology since, with some, such as biologics, viscosity increases with increasing drug concentration. These and other potentially competing effects make optimising rheology one of the most complex and critical aspects of formulation development.
Conventional Aqueous Nasal Spray or Powder Formulation
Although there are far fewer commercialised dry powder products, the benefits are, arguably, compelling, particularly for high-value or highly concentrated systems. Advantages include a longer shelf life, better retention times, potential for higher drug loadings, the ability to control aerosol properties for precise targeting and the relative ease of incorporating multiple, synergistic formulation enhancers. Set against these benefits are the drawbacks of greater manufacturing complexity, the need for drug dissolution in vivo and more limited device choice. Spray drying or freeze-spray drying are becoming increasingly explored for IN drug delivery. These processes allow for the use of a wide selection of formulation enhancers (e.g. co-solvents) to aid complex formulation preparations that would not be viable with a liquid system.
“There is considerable potential to deliver efficacious, patient-friendly solutions where there is a currently unmet medical need.”
Muco-adhesives, Mucopenetrants and Permeation Enhancers
These excipients tackle discrete tasks but have chemical and physical properties that need to be carefully balanced in the context of other constituents in the formulation. Muco-adhesives tend to be positively charged to promote interaction with negatively charged mucins and aid retention, helping to combat mucociliary clearance. In contrast, mucopenetrants enhance permeation through to the cell surface. When targeting the olfactory nerve, rapid penetration of the mucus is the principal concern, increasing the need for effective mucopenetrants. Existing muco-adhesive polymers are being explored for this application, along with strategies based on modification of the ionic strength of the localised environment. Where drug delivery is to the turbinates, permeation enhancers may be additionally required to, for example, disrupt tight junctions and enable access to the trigeminal neurons following successful transport to the epithelial surface.
Examples of formulation enhancers include surfactants, such as polysorbates or alkylsaccharides, polymers and oligosaccharides, such as chitosan, and cyclodextrin (CD) derivatives. Polysorbates and alkylsaccharides can have both hydrophilic and hydrophobic properties, allowing for stabilising interactions with multiple types of drug substances as well as the local nasal environment. Chitosan, on the other hand, is a cationic polymer, which means it can interact electrostatically with mucin to improve muco-adhesion, and also ionically with Ca2+ channels to open tight junctions and aid permeation. CD derivatives are widely used as permeation enhancers because of their ability to form inclusion complexes or nanoaggregates and the presence of both hydrophobic and hydrophilic groups in their cone structures.
Optimising the use of formulation enhancers is complex and the associated formulation science is advancing rapidly, making external advice and support in this area particularly valuable. Multiple studies are in progress to assess the synergistic effects of different combinations and ultimately this is most likely to be the most fruitful approach. A full discussion of this topic is beyond the scope of this article, but it is important to also highlight growing interest in the use of nanoparticles (NPs) for nose-to-brain delivery.
NPs typically either encapsulate or conjugate to the drug substance, shielding the molecule from the nasal environment and essentially changing its properties until it is finally released at the site of action. Some of the benefits include improving drug loading and stability, and the ability to essentially reverse the hydrophilic versus hydrophobic nature of the drug substance at different stages of the delivery process and/or to change surface charge to facilitate penetration through mucus and subsequent interaction with tight junction ion channels. Nanoparticles can protect drugs from degradation and more easily permeate through the mucus partly due to their small size, but are prone to aggregation and instability themselves, creating a new formulation challenge.
LOOKING FORWARD
The future looks bright for nasal drug delivery, which holds promise for tackling illnesses of pressing societal concern. There is considerable potential to deliver efficacious, patient-friendly solutions where there is a currently unmet medical need and to benefit from a corresponding reduction in the currently escalating costs of associated healthcare. However, realising this vision will be demanding. The development of nasal drug products for nose-to-brain delivery relies on a better understanding of the biology of the nose, enhanced device performance, advanced formulation expertise and tools to improve patient technique and compliance. Aptar Pharma is working across all these areas to help customers meet the challenge of effective nose-to-brain drug delivery (Figures 4 and 5).

Figure 4: Aptar Pharma has an unrivalled nasal device portfolio.
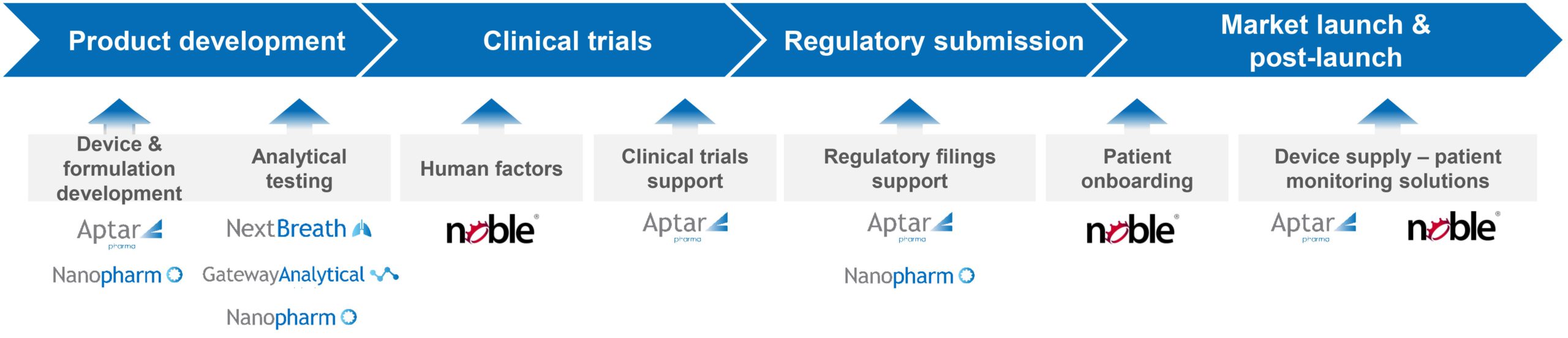
Figure 5: Aptar Pharma offers expert support from early-stage development to commercialisation.
For more information about Aptar Pharma and Nose to Brain, visit: www.aptar.com/resources/step-to-success-in-nose-to-brain-drug-delivery
REFERENCES
- “Naloxone Spray Global Market Report 2021: COVID-19 Implications And Growth”. Press Release, ReportLinker, Oct 2021.
- Image reproduced from Bioorg Med Chem, 2018, Vol 26(10), pp 2888–2905.

