To Issue 139
Citation: Birkhoff M, “Sustainability Measures are the Key to Meeting ESG Commitments”. ONdrugDelivery, Issue 139 (Oct/Nov 2022), pp 35–40.
Matthias Birkhoff and Christophe Marie discuss the advances Aptar Pharma has made in delivering on its sustainability objectives.
“Pharmaceutical drug delivery solution manufacturers, such as Aptar Pharma, must address the calls to address climate change and incorporate other sustainable benefits into their products, all while maintaining patient safety and compliance within a strict regulatory environment.”
Climate change is a challenge that impacts us all. Around 70% of the world economy is now covered by net zero targets,1 with most companies having some form of climate strategy or commitment in place. However, without a clear focus and meaningful measurement standards, there is a risk that such plans will result only in empty promises and greenwashing. In fact, the New Climate Institute reviewed the environmental strategies of 25 global companies and found that their climate pledges were often ambiguous and actual emission reduction commitments were limited.2 Therefore, the question must be how to reach those goals and fulfil what has been agreed.
Overall, one clear and overarching target has been set – 1.5°C. That’s the global climate change goal world leaders have agreed to strive for. By limiting the planet’s warming to 1.5°C, or 2.7°F, by 2100, the hope is to stave off severe climate disruptions.
It is not just politicians that are driving change; environmental stability is becoming an increasingly high priority for consumers as well. In fact, over the past five years, there has been a 71% rise in online searches for sustainable goods globally, according to the Economist Intelligence Unit.3 In addition to climate change, consumers have placed more emphasis on how products can be made more sustainable by design and through recycling.
In a 2022 Aptar Pharma survey, conducted with German, French and American participants, 77% of 840 respondents indicated that it was “Important” or “Very Important” that the products they buy can be recycled (Figure 1). In addition, six of 10 respondents noted that they consider the recyclability of products when making purchase decisions. A total of 70% of respondents also said that they were willing to pay more for a product that they could recycle. In summary, these responses clearly demonstrate that public opinion has started to shift towards demanding more sustainability initiatives that protect the environment.
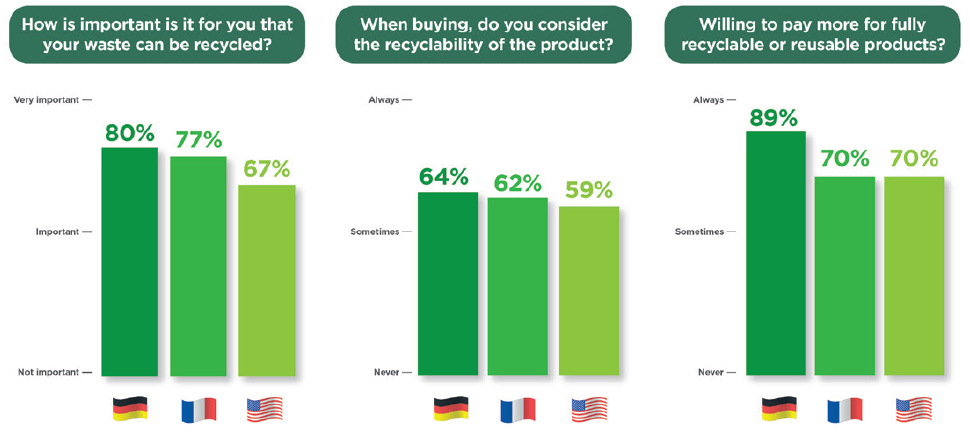
Figure 1: International research on consumer sentiments on recyclability and reusability.
Pharmaceutical drug delivery solution manufacturers, such as Aptar Pharma, must address the calls to address climate change and incorporate other sustainable benefits into their products, all while maintaining patient safety and compliance within a strict regulatory environment. Interestingly, the pharmaceutical industry appears to be taking on more ambitious objectives than most, committing to a reduction in emissions of 45.8% in 12 years compared with an average target of 44.6% across other industries in the same timeframe.4
By all means, the industry has good reasons to be ambitious. In 2018, researchers at McMaster University (Hamilton, Canada) conducted a study of CO2 emission levels generated by the automotive industry compared with the pharmaceutical industry. The study considered the direct emissions generated by operations and indirect emissions created by the energy purchased by the industry. The researchers found that the pharmaceutical industry generated 52 megatons of CO2 emissions in one year, compared with 46.5 megatons generated by the automotive industry over the same period.5 Achieving the goals that the pharmaceutical industry has committed to will require a concerted effort across the entire value chain, as challenges in this highly regulated industry remain increasingly complex. All this requires close study of the current products and an understanding of their environmental impacts, which then allows companies to strategically optimise existing products or design even better new ones.
An immediate action taken by Aptar Pharma to address some of these complex issues has been to change the way it designs its products. As of 2022, Aptar Pharma has committed to design sustainability and circularity into new product development programmes. This could help position the company ahead of today’s tightening regulations and contribute to a more sustainable future.
To make new device design more effective, Aptar Pharma has co-developed an eco-design tool with Sphera (Fishburn, UK) that incorporates lifecycle assessment (LCA) perspectives to help design new products that minimise their impact on the environment. Sphera is a leading provider of environmental, social and governance (ESG) performance and risk management software, data and consulting services with operations around the world. The eco-design tool incorporates the assessment of inputs, outputs and the evaluation of a product’s environmental impact throughout its entire lifecycle. Calculating the CO2 footprint, recyclability and circularity are key measures that will help guide Aptar Pharma to design every new or improved drug delivery system to enhance its sustainability performance. The first step towards making progress on product development is often studying existing products more closely.
OPHTHALMIC SQUEEZE DISPENSER COMPARISON
One analysis-worthy example is Aptar Pharma’s ophthalmic squeeze dispenser (OSD), a preservative-free multidose eyedropper system. Such eyedropper systems offer a convenient alternative to the single-use vials that are widely used for ophthalmic treatments. By closely studying the OSD’s use, properties and how it impacts the environment, it is possible to support better product selection choices between drug delivery options.
Aptar Pharma recently conducted a study that compared its OSD for preservative-free formulations, with single-use blow-fill-seal (BFS) eye drops on a number of sustainability measures. The study compared only the OSD and BFS formats of a specific commercially available eye-care product, allowing for a direct comparison. Due to the complexity of the entire product lifecycle, Aptar Pharma focused on three measures for the same dosing regimen: plastic waste, CO2 impact and formulation waste volumes. This study did not assess additional factors, such as energy consumption in manufacturing, resin transportation and other factors in its calculations, on the assumption that these other aspects would be largely net comparable on a global warming potential (GWP) basis and for simplicity of comparison.
Plastic Material Reduction With Aptar Pharma’s OSD
A single multidose OSD unit typically contains 10 mL of formulation liquid. A typical selling unit for single-use BFS vials would include 20 ampoules of 0.3 mL fill volume each (for a total formulation volume of 6 mL). To perform a meaningful analysis of equivalent plastic material use, the impact of formulation waste and CO2 on the data must first be normalised.
A single OSD unit containing 10 mL of formulation weighs 6.2 g. The same 10 mL of formulation packaged into single-use BFS plastic vials requires just over 33 vials, which would weigh 37.2 g. The majority of this weight is attributable to the plastic vial materials. Therefore, 10 mL of an eye-care medication packed in BFS vials uses five times more primary packaging material than the same formulation volume packaged in an OSD multidose eyedropper. Because of this sizeable weight difference and based on Aptar Pharma’s LCA calculations (made with Aptar Pharma’s eco-design LCA tool, no third-party review was conducted and secondary packaging/cartonnage were not considered), this translates into a CO2 impact for the OSD that is six times lower than that of the BFS equivalents.
By extension, at a commercial scale, based on comparable 10 mL fill volumes, 100,000 of Aptar Pharma’s OSD devices are equivalent to 3.3 million BFS single-use vials (1 OSD: 33 BFS). At this scale and by this measure, 100,000 OSDs would save more than 8,000 kg of CO2 over the BFS format (Figure 2), which is the equivalent amount of CO2 generated by more than five round-trip flights between Paris, France, and Beijing, China (82,000+ km total distance).6 This demonstrated reduction in primary packaging materials using the OSD option also translates into reduced pack sizes, which reduces the number of pallets and shipping volume needed to ship the product, which will also result in reduced carbon emissions generated through the transportation process.
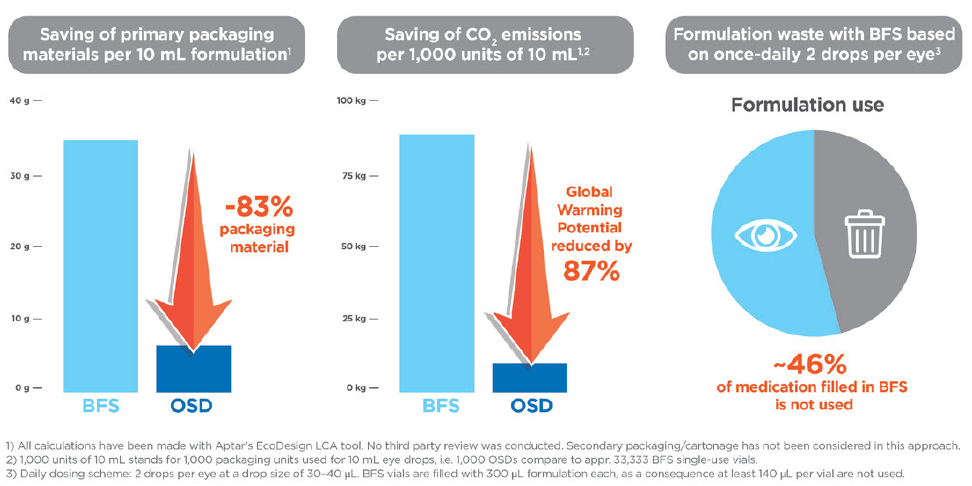
Figure 2: Comparison of packaging material waste, CO2 emissions and formulation waste between Aptar Pharma OSD and BFS single-use vials.
“This drive to improve Aptar Pharma’s devices through innovative design for recyclability has led to favourable ratings from cyclos-HTP for a number of its systems.”
Minimising Formulation Waste With Aptar Pharma’s OSD
Aptar Pharma also assessed the difference in the amount of formulation solution wasted with both packaging options. It found that OSD devices provide environmental advantages over BFS with respect to minimising formulation waste. A typical dosing regimen for an eye condition could be two drops per eye per day, for as long as 60 days, which was the model for this comparison. A typical drop size volume for either eye-drop dispenser type would be approximately 40 μL per drop. Therefore, patients would use 160 μL of formulation delivered as drops per day, for a total of 9.6 mL used over the course of the entire 60-day treatment.
One major difference between OSD and BFS vials is that each BFS vial must be discarded immediately after opening and administration because the BFS vial cannot maintain formulation sterility or protect against contamination. Each BFS vial is filled to 300 μL, with 140 μL of formulation discarded as waste from each vial (Figure 2). Aptar Pharma’s OSD device deploys a mechanical tip seal to protect the contents of the multi-use unit from external contamination and can safely administer 250 doses from a single unit with a single 10 mL fill.
As BFS vials are sold in selling units of 20 vials, the patient would need to purchase three selling units, or 60 vials, to complete a 60-day treatment course, whereas with the OSD, only one dropper bottle would be required to complete the entire treatment. Additionally, the OSD has a high-evacuation rate, delivering virtually all its 10 mL fill volume without waste.
Therefore, over the course of the 60-day treatment regimen, the BFS vials would result in 140 μL per vial, a total of 8.4 mL, of formulation solution being discarded as waste, whereas virtually none (1–5 μL) of the formulation would be wasted from the single OSD device. When scaled to 100,000 OSD units (which is equivalent to 3.3 million BFS vials), the total additional formulation waste volume produced by BFS vials is more than 460 L of formulation solution.
A single OSD device with 10 mL fill provides access to 67% more formulation and three times the treatment duration per selling unit compared with BFS vials (20 vials per package). In terms of the primary packaging materials, the treatment course requires 60 BFS vials versus a single OSD device, and almost 10 times more packaging material is consumed through the BFS packaging option. In summary, Aptar Pharma’s multidose OSD eye-drop device provides substantial savings in plastic and formulation waste, as well as a reduced CO2 footprint, based on Aptar Pharma’s comparative analysis.
This OSD comparison serves as a strong example of how much can be done to reduce environmental damage through the data-driven selection of a device option that meets the same drug delivery requirements but reduces waste and increases efficiency. Imagine how much more can be done now that Aptar Pharma has dedicated itself to optimising existing devices and designing all new devices with sustainability and circularity objectives as primary drivers. The company has successfully implemented these types of initiatives with a range of different product lines towards meeting Aptar Pharma’s broader long-term environmental objectives. The following are some examples of how Aptar Pharma has been successful in optimising its existing products or services, and how it has started to design circularity and sustainability into the new devices it makes.
pMDIs AND SUSTAINABLE PROPELLANTS
Propellants used in medical devices have come under new and tighter regulations designed to reduce their environmental and GWP impact. Aptar Pharma recognised some time ago that propellants presented a problem that needed to be addressed. As a result, it engaged in supporting its clients who were researching new propellant options that are suitable for Aptar Pharma’s pressurised metered dose inhaler (pMDI) devices and would reduce the overall GWP. Companies are targeting the reduction of commonly used propellants, such as hydrofluoroalkanes (HFAs) and hydrofluorocarbons (HFCs), by 85% by 2047, as they have been designated as restricted materials in the Kigali Amendment (2016) to the Montreal Protocol. The challenge with introducing new propellants is that both patient safety and functional drug delivery aspects must be proven in studies before existing products can be converted to using a new propellant.
HFA-152a and HFO-1234ze have been identified as two potential propellants with a considerably lower GWP and are therefore currently under assessment at Aptar Pharma. HFA-152a has undergone extensive study, including full inhalation toxicology studies, and has shown strong results with no adverse findings to date. By replacing the current propellant, HFA-134a, with HFA-152a, there is the potential to reduce climate change and global warming impacts of inhalers in the UK by 90%–92%, according to a study by the University of Manchester (UK). HFO-1234ze has the potential to deliver an even greater GWP reduction of 99%, but safety and toxicology data available in the public domain are currently limited. Aptar Pharma remains committed to supporting its customers with a range of services to help de-risk and accelerate their low GWP pMDI programmes, including the optimisation of a new metering valve technology platform that demonstrates improved chemical and functional compatibility with both HFA-152a and HFO-1234ze propellants.
RECYCLABLE SYSTEMS BY DESIGN
Historically, recyclability was not a primary consideration in the development of drug delivery devices. The primary focus was always to create devices that reliably and safely delivered the required drug dosages to the patient with convenience. Today, those functional parameters are still just as important but now improving the recyclability of the devices during the design stage has taken on significantly more importance.
This drive to improve Aptar Pharma’s devices through innovative design for recyclability has led to favourable ratings from cyclos-HTP (Aachen, Germany) for a number of its systems. These recyclability improvements include designing mono-material systems that enable simple and complete recycling of devices without compromising functionality or safety. Aptar Pharma has also shifted to using medical-grade source materials for pharmaceutical applications that support recycling capabilities. Higher levels of recyclability can also be achieved by eliminating harder-to-recycle device component materials, such as metals, so that the entire device can be recycled without significant intervention. Aptar Pharma has looked at a variety of ways to enhance the recyclability of its products and invested in making these opportunities a reality across a number of its product lines.
The Recyclable Nasal Spray Pump
Aptar Pharma is currently developing a new recyclable nasal spray pump that incorporates a mechanical tip seal feature for preservative-free multidose formulations. Any metal components of the spray mechanism have been removed, and the innovative technical design is based on plastic components only. The full plastic spray pump thereby achieves very high recyclability ratings and is specifically designed for use with nasal saline or comparable formulations.
“Aptar Pharma has successfully begun to implement a mass balance
accounting system to increase the use of more sustainable inputs,
such as bio- or circular feedstocks, that could reduce its CO2 footprint.”
Proventu Mono-Material Tube Systems
Aptar Pharma has developed its first mono-material tube system, called Proventu, which was designed to meet pharma product standards using an eco-design approach. All device components are made entirely of medical-grade polypropylene, enhancing the recyclability of the system to full recyclability. The mono-material device can be placed directly into existing recycling streams when emptied after use. This is just one example of Aptar Pharma’s drive to implement mono-material system designs to enhance the recyclability of its products and have a positive impact on the environment.
Rated for Recyclability
Aptar Pharma’s Airless+ dermal drug delivery systems use the company’s eco-design tools to meet the tightened US Pharmacopeia <661> regulations, excluding metal parts and using medical-grade resins. The Airless+ system efficiently dispenses its dermal formulation leaving only minimal volumes when fully used, making the device easy to recycle directly in existing recycling streams without further preparation. It achieved a “Class AAA” with an “excellent recyclability” rate of 96%–98% as rated by cyclos-HTP.
Aptar Pharma’s bag-on-valve (BOV) continuous dispensing systems are another example of designing recyclability into the device. The BOV system can include recyclable aluminium, removable actuators and offers high product evacuation rates making them easily recyclable. Designed to use compressed air/nitrogen propellant systems, they reduce greenhouse gas emissions compared with othercommon propellants found in similar applications. The BOV systems achieved a “good recyclability” rating (Class A) of the raw packaging assembly from cyclos-HTP.
Both these examples demonstrate the importance of designing recyclability into devices from the start. This enables the device to be easily recycled and reduces the environmental impact of the product across its entire lifecycle.
THE IMPORTANCE OF MASS BALANCE
Aptar Pharma has also taken more holistic approaches to reduce its carbon footprint in a sustainable and responsible way. Aptar Pharma has successfully begun to implement a mass-balance accounting system to increase the use of more sustainable inputs, such as bio- or circular feedstocks, that could reduce its CO2 footprint. Aptar Pharma has already implemented massbalance systems at some of its sites.
Mass balance is a system designed to document and track the flow of materials through the value chain, including the amount and sustainability characteristics of circular and/or biobased content in products. This approach gives Aptar Pharma control over material composition and supports claims about device material composition through the associated documentation and records. Several Aptar Pharma production sites have implemented a mass-balance system and achieved ISCC certification,7 allowing them to incorporate renewable feedstocks into production. A mass-balance system allows a company to maintain better data regarding material flows and allows for more flexibility around controlling feedstock profiles, producing more sustainable products. Aptar Pharma is expanding the mass-balance approach and ISCC certification to additional sites across its network to achieve even greater sustainability improvements.
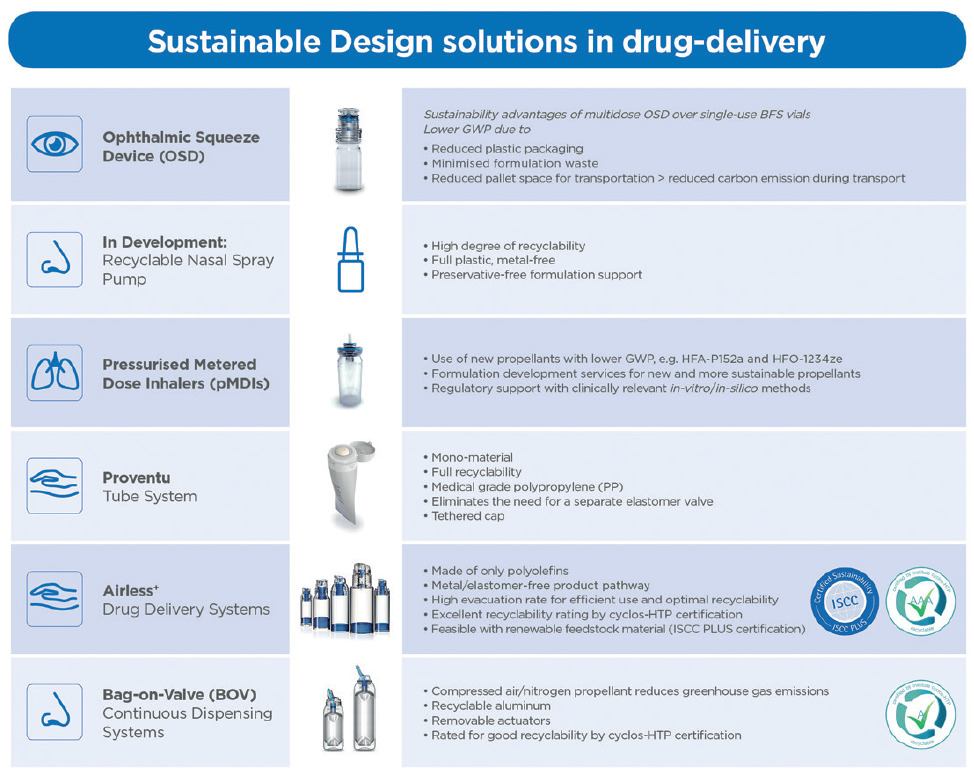
Figure 3: Overview of Aptar Pharma’s product solutions, supporting sustainability targets in drug delivery.
CONCLUSION
Aptar Pharma has successfully developed drug delivery systems that reduce raw material and plastic waste, offer enhanced recyclability, reduced or replaced inputs and minimised formulation waste. It has also successfully instituted new systems and philosophies, such as mass balance and the circular economy across its business that will enable the company to measure and improve on the successful achievements of its environmental objectives to date (Figure 3). As a market leader in pharmaceutical drug delivery systems, Aptar Pharma has demonstrated that a companywide strategic effort can quickly produce positive results for the environment. Its commitment to the environment is long term and there is still much more to do. Aptar Pharma will continue advancing its sustainability objectives while maintaining the highest standards of quality and functionality for its products and services, ultimately enabling patients around the world to achieve better and healthier lives.
Aptar Pharma strives to maintain industry leadership in pharmaceutical drug delivery technologies while also taking an emerging leadership position in the industry for its environmental and sustainability efforts. What could be more important than improving people’s health and the planet we all share together?
For more information on Sustainability and Aptar Pharma, visit: www.aptar.com/resources/sustainability-measures-are-the-key-to-meeting-esg-commitments
REFERENCES
- “COP26 Explained”. UN Climate Change Conference UK 2021, Jul 2021.
- “Corporate Climate Responsibility Monitor 2022”. New Climate Institute, Feb 2022.
- Martins A, “Most Consumers Want Sustainable Products and Packaging”. Business News Daily, Aug 2022.
- “Companies Taking Action”. Science Based Targets webpage, Accessed Oct 2022.
- Belkhir L, Elmeligi A, “Carbon Footprint of the Global Pharmaceutical Industry and Relative Impact of its Major Players”. JCLP, 2019, Vol 214, pp 185–194.
- “ICAO Carbon Emissions Calculator”. ICAO webpage, Accessed Oct 2022.
- “ISCC Certificates”. ISCC webpage, Accessed Oct 2022.

