To Issue 148
Citation: “Product Showcase: Optimising Gastric Resistance in Complex Oral Drug Products with Novel, Functional, Ready-To-Fill Capsules.” ONdrugDelivery, Issue 148 (May/Jun 2023), pp 20–21.
“Capsules are relatively simple to use compared with tablets, which require more formulation development and quality control and therefore take longer to produce.”
Hard capsules are one of the most common solid dosage forms used for oral administration of active ingredients. Capsules are relatively simple to use compared with tablets, which require more formulation development and quality control and therefore take longer to produce. Capsules can be manufactured in different sizes and using different materials, depending on their main purpose and content. Hydroxypropyl methylcellulose (HPMC)-based capsules are the most common non-animal-derived alternative. Although they are a good substitute for gelatin, they have limitations, particularly when intended for enteric formulations because they are not resistant to gastric acid.
Coating capsules with functional polymers is a potential solution to this challenge. However, for the growing number of active molecules that are sensitive to acid, moisture and temperature, such as nucleotides, peptides and live biotherapeutics, the coating process conditions are unsuitable because these processes can cause damage or degradation.
Therefore, a new approach is necessary for the oral delivery of sensitive actives that enables reliable protection and provides an efficient and accelerated drug development timeline. Developing a delayed-release formulation of acid-sensitive actives that can be used in solid dosage forms, such as tablets, pellets and capsules, can be costly. Typically, this involves several stages, starting from R&D to scale-up and validation, all of which require significant investments of time and money. Sourcing a ready-to-fill functional capsule is an effective way of saving costs, reducing time to market and boosting the performance of oral drug delivery products.
“Developing a delayed-release formulation of acid-sensitive actives that can be used in solid dosage forms, such as tablets, pellets and capsules, can be costly.”
Evonik’s EUDRACAP® is a non-animal-derived platform of functional, ready-to-fill capsules for fast-track development of sensitive drugs. The HPMC capsules are functionalised with a coating based on EUDRAGIT® polymers and can be easily opened and closed on standard manual and automatic capsule filling systems.
AN EFFECTIVE SOLUTION FOR ENTERIC DRUG DELIVERY
EUDRACAP® capsules allow targeted drug delivery (also of sensitive actives), reduce clinical risk and accelerate time to market. One type of capsule in the EUDRACAP® portfolio is EUDRACAP® enteric, which is designed to optimise gastric resistance and improve absorption of drug products targeted for release in the upper small intestine. The high-quality HPMC capsules feature a precisely tailored functional coating that is well accepted by many key regulatory bodies around the world.
EASY TO OPEN AND CLOSE ON STANDARD EQUIPMENT
EUDRACAP® enteric capsules can be easily opened and closed on standard manual and automatic capsule filling systems. Various forms can be efficiently filled, including powders, pellets, granules and selected liquids (Figure 1).
Figure 1: SEM image of final locked capsule at 20x magnification.
RELIABLE PROTECTION AND PRECISE, RAPID RELEASE
Dissolution testing confirms that no drug release occurs during the acid stage (HCI 0.1N). After conversion to the buffer stage at pH 6.8, rapid release begins immediately, with excellent reproducibility across all tested batches (Figure 2)
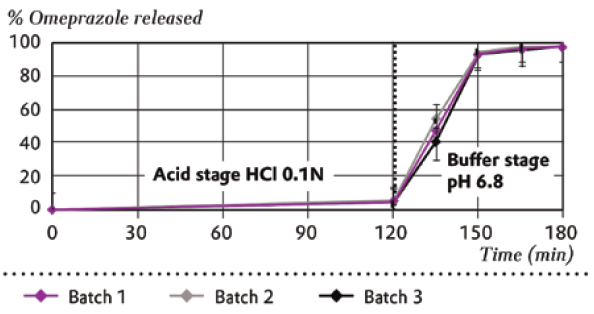
Figure 2: Dissolution profile using omeprazole as an acid labile model compound.
.
Using EUDRACAP® enteric ready-to-fill capsules can accelerate the product development process, reduce complexity and risks during formulation development, and simplify scale-up and validation by replacing several complex process activities with just a single capsule-filling step.
In addition to EUDRACAP® enteric capsules, the EUDRACAP® portfolio also includes a customisable product, EUDRACAP® Select, which allows a capsule to be tailored to the specific needs of the pharmaceutical product.

