To Issue 159
Citation: Monk A, Krista K, Montagnetegy C, Schanté C, “Leveraging AI and Big Data for Eco-Design Collaboration in Pharma”. ONdrugDelivery, Issue 159 (Apr/May 2024), pp 17–22.
Alissa Monk, Kami Krista , Cédric Montagnetegy and Carole Schanté, explore the potential of artificial intelligence and big data in eco-design with the aim of transforming the pharmaceutical industry towards greater sustainability.
“To realise the potential of software for transforming the pharmaceutical industry’s core business – both development and, ultimately, manufacturing – to become more sustainable, pharma companies need to switch into collaboration mode.”
Sustainability and digitalisation are currently two of the most significant drivers of change for companies globally, regardless of sector. Yet, until recently, the two had not converged to leverage the latest advances in technology to accelerate the transition to a more sustainable age. There is now ballooning pressure on companies to measure, set targets, publicly report and, most importantly, act on sustainability issues, something that was previously often neglected and seen as a nice-to-have by many organisations. In particular, the pressures – and, in some cases, requirements – on companies to transform the core business, together with their suppliers, to become more sustainable is creating a whole new set of challenges that require unique processes, tools and skillsets to master.
The pharmaceutical industry and its suppliers are especially vulnerable to triggering intractable complexity with this endeavour, given the regulatory restrictions, supply chain intricacies and technical delicacies involved. Software, leveraging the latest advances in artificial intelligence (AI) and big data, promises to reduce the activation energy and sustainability expertise necessary to really embrace sustainability, identify patterns and extract insights in large volumes from unstructured and diverse data, manage complex data sharing needs across supply chains and so much more.
To realise the potential of software for transforming the pharmaceutical industry’s core business – both in development and, ultimately, manufacturing – to become more sustainable, pharma companies need to switch into collaboration mode with start-ups and with other stakeholders in the value chain and industry.
There have been recent directives developed to align reporting frameworks, defining reporting disclosure standards and bringing greater scrutiny to claims and data. There is also an expectation that companies report not only on their direct operational impacts, but also on value chain impacts. Currently, this is usually an arduous and resource-intensive task, so there is a significant opportunity and need to automate the reporting process using digital advancements to free up the expertise (and, for many, budget) within companies to focus on reducing negative impacts at the speed at which both the planet and society need.
A variety of procurement pressures and regulatory developments in Europe are now shifting the focus for companies from exclusively reporting on their status quo to making actual progress on their sustainability goals – a trend that has also triggered unique pressures for the pharmaceutical industry. What stands out the most for pharma is that hospital procurement processes and tender award assessments are changing to increasingly include sustainability as a key decision criterion, which is driven by regulations (in the Nordics, sustainability is already weighted at 30%).1
Some of the most immediately important regulations for pharma are the EU Empowering Consumers for the Green Transition (adopted),2 EU Green Claims Directive (position adopted by EU Parliament),3 the Industrial Emissions Directive (adopted),4 Environmental Crime Directive (adopted),5 Packaging Regulation Revision (provisional agreement)6 and, most recently, the Corporate Sustainability Due Diligence Directive (adopted by Legal Affairs Committee).7 Hospital tenders account for ~40% of drug purchases in Europe.8 MedTech tenders with environmental, social and governance (ESG) criteria have grown with a compound annual growth rate of 7.5%, the equivalent of 414 contract notices with ESG criteria for medical devices and 241 for medicines in 2020 – which showed an overall dip in tenders.9
Countries across Europe are increasingly implementing procurement processes with sustainability weightings, including Norway, Denmark, the Netherlands, Iceland, Sweden, the UK, soon Portugal, France and, to some extent, Germany.10–14 This means that, for any drugs in development today, their reimbursement and competitiveness in the market depends on their actual sustainability performance. On the other hand, for drugs with otherwise already expired patent protection, this may create an interesting incentive for re-designing manufacturing processes for drugs already on the market.
“For the pharmaceutical industry to make progress and achieve the targets set or required, every scientist and engineer involved in process design needs to consider sustainability as a design criterion.”
What does all this mean? Alongside these regulations, the pressures of hospital procurement and the need to consider value chain impacts, there is also the pressure of advancing sustainability progress at a much faster rate. To address this, companies have been moving towards integrating sustainability into the product or process design – “eco-design”. When considering the sustainability of a product or process, it is, in the long run, vital to understand the environmental footprint of its full lifecycle to reliably identify the factors of the product or process that drive its environmental impacts. This is usually done using a lifecycle assessment, a particularly laborious task that requires high expertise, software, a vast amount of time and, usually, high costs. While these are an accurate and informative assessments, for many companies, the time requirements (and usually costs) make this an “unsustainable” method to gain the information needed to inform decisions and strategy, particularly given the pressures to move at an accelerated pace.
All of this together signals a need for specialist software that will allow for rapid environmental impact assessments that ease decision making, set an achievable strategy and allow directionally correct signals about the environmental impact of different decisions to be extracted. Herein follows the move to integrate AI, big data and modelling of product and process design to enable every scientist, process engineer and other decision maker involved to seamlessly consider sustainability as a design criterion and apply eco-design into the fabric of their process development.
ECO-DESIGN
For the pharmaceutical industry to make progress and achieve the targets set or required, every scientist and engineer involved in process design needs to consider sustainability as a design criterion. It is estimated that 70%–80% of the final environmental impact of a drug is determined during the first few years of process design,15 although a drug takes, on average, 12 years to get to market.16 As a result, scientists and process engineers at that early stage are the first line of defence. Sustainability needs to move from being a topic that individual leaders, such as chief sustainability officers, or corporate teams are responsible for, to being a decision criterion that is considered by every employee, every scientist and every engineer.
However, sustainability expertise is rarely mastered by every scientist or engineer, which – in addition to facing ballooning information complexity and limited resources or support by middle and senior management – currently makes it almost impossible to embed sustainability into every decision process upfront, especially in larger firms. Designing processes is already complex, given the scientific, technical and quality criteria scientists have to work with. Adding sustainability ends up exceeding the timescale available for making a particular process design decision due to compounding complexity (Figure 1):
- The key performance indicator “sustainability” actually consists of a plethora of interconnected decision criteria (emissions, water, waste, resource usage, toxicity, etc). Each decision usually brings trade-offs between the different sustainability criteria.
- For each sustainability criterion, the sources of negative impacts in a process tend to be spread out, rather than being concentrated in an isolated part.
- For each impact source, the solution landscape – consisting of more sustainable process inputs or methodological blueprints – is opaque, requires diverse high-volume data sources to assess and changes frequently.
- It will be key to carefully balance sustainability versus other output criteria, such as cost. Often, such an approach may not be supported by company management, and both a clear mindset change and increasing regulation will be beneficial.
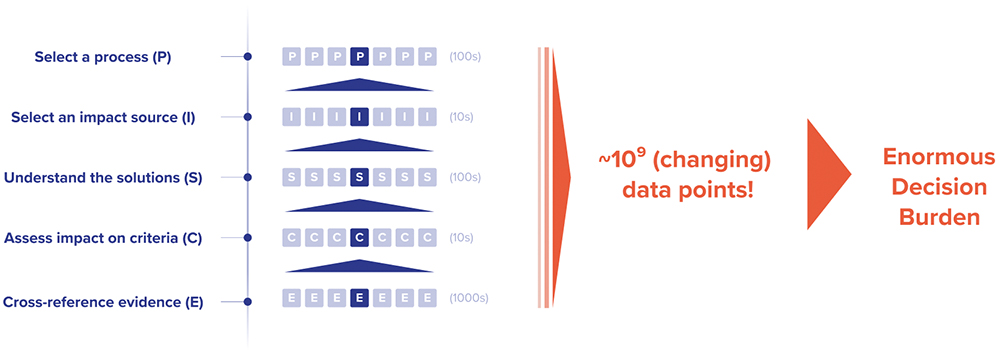
Figure 1: The ballooning complexity of eco-design.
Given the nature of R&D, iterative development and decision making, feedback loops are the norm. Thus, not only are scientists and engineers faced with this complexity once, they need to re-evaluate their decisions easily, including considering new solutions on the market or those that have been identified in research since, which is currently an impossible task.
An additional point to consider is that scientists and engineers are often under time pressure to work and frequently need to make decisions without having the time or the knowledge to bring in these sustainability considerations, especially for routine work or ordering materials that they are used to working with. Companies do not necessarily have the “sustainability mindset” ingrained in each individual, especially where decisions can be made “by default”.
To consider sustainability seamlessly and continuously, companies would significantly benefit from software tools, supported by AI and big data, that, ideally, are not exclusively specialised on sustainability: decision makers in companies – across all levels – benefit from integrating sustainability into their daily decisions. On the one hand, software is excellent at organising and processing large quantities of data at scale and keeping it up to date, while different forms of AI are enabling the recognition of otherwise hidden patterns and connection of information points to build a higher order reasoning chain about this information and “understand” it at scale.
“Software tools built with AI and big data can therefore augment the existing specialised expertise of the scientist and help to increase the iteration cycles of a particular decision.”
One of the aspects that makes assessing the sustainability profile of decisions so difficult is that exact data for the specific variation of the decision that a scientist is evaluating usually does not exist. Instead, there are many different data points that need to be “stitched together” and pattern-matched based on the specific conditions for which it was originally provided. This is necessary to make an informed assessment of its relevance under the given circumstances. To do that, it requires scraping or ingesting, processing and then analysing large amounts of data with the help of AI, such as natural language processing for text.
Software tools built with AI and big data can therefore augment the existing specialised expertise of the scientist and help to increase the iteration cycles of a particular decision. Since a multitude of aspects about a process can be changed, and these changes are not necessarily isolated from one another, leveraging computational power to assess a combinatorial option space to enable scenario modelling becomes one of the most powerful applications for unlocking the potential of sustainable design.
To unlock the potential of eco-design, collaboration between stakeholders across the value chain of a product is critical. The ability for downstream stakeholders to design their part of the value chain more sustainably is dependent on choices made upstream and the availability of process inputs to enable the successful implementation of more sustainable methodologies. As a result, to align stakeholders successfully, data-sharing practices become key. This not only fosters a more rapid and actionable dissemination of information about the available solutions but also enables feedback mechanisms to be created between suppliers and customers when existing offers are not sufficient. Given the sensitivity companies have around their data, it becomes a necessity to provide a streamlined and high-security data-sharing infrastructure that enables them to selectively share data with specific individuals at specific companies (e.g. their verified customers).
While a decision-making platform for eco-design and a data-sharing infrastructure are foundational, it is in no way an exhaustive illustration of how digital tools can support rapid progress towards a more sustainable age in the pharmaceutical industry. For example, there are many more tools specialised to specific tasks, such as improving the operational energy efficiency of machinery used, sorting waste effectively, verifying the source of materials (digital material passports) and much more, that have not yet been considered.
TEN23 HEALTH & ELIO CASE STUDY
Co-creation through a design partnership is an excellent way to engage with and help design these necessary digital solutions with software companies where vision is aligned. Given the need for collaboration, no single company within the pharmaceutical value chain can build and provide the most valuable platforms and tools. These are necessary for embedding eco-design across the value chain and ensuring that reporting is not a burden, but rather an enabler of collaboration.
Equally, companies designing software solutions for the pharma industry might have brilliant new ideas and technology to implement, but they often lack a complete understanding about the intricacies of decision making inside a pharma company to develop tools that are both practical and usable. Thus, there is an opportunity for companies in the pharmaceutical value chain to work together with other companies, especially innovative start-ups building software to design the best solutions possible for the industry.
A great example of such a partnership in action is between the software start-up Elio and ten23 health.
ten23 health is a pharmaceutical service provider with an ambitious vision to create and inspire positive impact on the planet and on people. ten23 health believes that sustainability is more than just carbon and did not find any tools that met its needs – there was a need for software that incorporates various sustainability measures, reports a sustainability endpoint score that reflects real impact, allows the rapid impact assessment of a full product or process and offers alternatives. ten23 health was also interested in a solution that not only allows sustainability to be embedded in the way it operates but also has all employees able to seamlessly integrate it into their ways of working, thereby alleviating some employee engagement issues typically encountered.
Similar to ten23 health, Elio applies holistic thinking, is driven to create real impact and had a solid base on which to build a solution that would meet ten 23 health’s needs by using advanced digital technologies. As a relatively small company, ten23 health did not have the large budget that would allow it to pay for bespoke software to be developed. However, as the company is committed to creating a culture of collaboration and sharing, by partnering with Elio, ten23 health saw the opportunity to help other companies in the pharma and CDMO sector – as well as others – to advance their strategies and make significant progress on sustainability for the good of the planet and society.
“The biggest trap for a software start-up is to end up building highly bespoke solutions for each customer, rather than one system used by many companies.”
Elio is a software start-up that envisions helping to create a world where sustainable manufacturing has become the norm. The company believes that scientists and engineers – who are responsible for designing products and processes that ultimately determine their sustainability profile for years, if not decades – need to be empowered with the tools and authority to allow them to make the best decisions collectively, with sustainability seamlessly integrated into their day-to-day processes. Elio saw that ten23 health – together with the company’s expertise in drug development – was the best starting point for this vision. Since pharma is notoriously complex in its decision making and opaque from the outside, Elio was looking for a partner that would work with the company to lift the veil and ensure that the product they built was not just useful in theory but was also usable in practice, across different drug modalities and processes.
The biggest trap for a software start-up is to end up building highly bespoke solutions for each customer, rather than one system to be used by many companies. ten23 health understood how important it was for the joint success of both companies to make sure Elio served their specific needs, but not in a way that precluded use by other pharma companies. In fact, ten23 health welcomed the idea that this would not be exclusive to them and that they would help define a platform that others would join and co-ordinate through.
As of March 2024, ten23 health and Elio have successfully completed the first phase of their design partnership, which has focused on specifically supporting the selection of more sustainable consumables used for pharmaceutical development and manufacture. The first version of the tool allows a user to understand the sustainability profile of a consumable, based on a holistic score. However, ten 23 health and Elio’s work together does not end here. The two companies will continue to work together to get closer to the end vision of designing the entire pharma development and manufacturing process more sustainably.
When considering what sort of digital tools or features these tools may be missing for the sustainability journey, it is advisable to consider proactively working together with a software start-up. The value for pharma companies – of almost any size and constellation – in working with innovative start-ups in design partnerships for digital solutions is very clear:
- Shape the solution to specific needs: Every pharma company has certain needs that are more prominent compared with others. Working at the earliest stages with, for example, a start-up to design a solution provides the opportunity to prioritise the most prominent needs. A product roadmap may end up servicing all future needs, but through a design partnership, the roadmap can be reordered and, thereby, the speed with which the product solves or considers specific needs.
- Cheapest development and lowest risk: Investors finance start-ups to build the first version of the product, which effectively subsidises the innovation and the process of solving the company’s needs.
- The gift that keeps on giving: The company gains early access to the solution that, in the long run, grows in value without having to invest to build and maintain it, as the cost is spread across future customers of the solution.
CONCLUSION
Evidently, the pharma industry is at a point where the convergence of sustainability and digitalisation could enable the automation of tasks such as reporting and eases the burden of tasks such as assessment and finding more sustainable alternatives to products and services. For everyone in the pharmaceutical supply chain to be able to benefit fully from these tools, it is necessary to adopt a mindset of sustainability focus, collaboration and sharing.
Since the environmental impact of a drug is spread out over its entire value chain and choices made by upstream stakeholders limit the potential of those downstream to design a drug and its manufacturing processes more sustainably, collaboration becomes a critically necessary feature of eco-design.
To scale the matchmaking between pharma companies and solution providers (software and beyond) and bring the full benefit of collaboration to bear, it cannot just be an ad hoc, occasional action. It needs to be much more structurally integrated into the way sustainability strategies are executed within companies. However, current forums that are meant to foster greater collaboration in the industry regarding sustainability more generally are not set up to build real, supportive solutions successfully for the entire industry. There are usually too many members, who are not necessarily all at the same critical stage, primed for action. Consequently, they fail to integrate companies that could help with implementing the necessary pre-competitive solutions and lack a general focus on building solutions over writing about them. For many that attend these forums, it feels like an endless cycle of debate clubs and white papers.
Thus, to advance this topic into the pharma sector, ten23 health and Elio have initiated a unique “implementation consortium” for eco-design in the pharmaceutical industry, which will kick off later in 2024. Through a very intentional structure that includes symbiotic industry-tech pairing, eco-design readiness level assessment and small member size for lean leverage, this partnership is ensuring that the focus of this group is on building – not writing about – solutions. The goal is to provide the space to pilot, workshop, improve and roll-out potential solutions for bringing eco-design to the pharmaceutical industry and its value chain. Members across the pharma value chain can come to the group to bring a problem or gap to members, initiate resource pooling projects with other industry members and build proof of concepts or case studies to then later roll out in traditional forums.
If you are ready to advance the roll-out of eco-design principles meaningfully within your company, and are wondering how to proceed from here, especially – but not only – if you are interested in joining the consortium, please contact Kami Krista, Chief Executive Officer, Elio ([email protected]), or Alissa Monk, Fairstainability Co-Circle Lead, ten23 health ([email protected]).
REFERENCES
- Conic F, Hosseini M, Buente M, “Will Environmental Sustainability hold sway over the Pharmaceuticals Industry?”. Roland Berger, Nov 2022.
- “MEPs adopt new law banning greenwashing and misleading product information”. Press Release, European Parliament, Jan 17, 2024.
- “Parliament wants to improve consumer protection against misleading claims”. Press Release, European Parliament, Mar 12, 2024.
- “Reducing pollution from industry and large livestock farms”. Press Release, European Parliament, Mar 12, 2024.
- “Environmental crimes: MEPs adopt extended list of offences and sanctions”. Press Release, European Parliament, Feb 27, 2024.
- “Deal on new rules for more sustainable packaging in the EU.” Press Release, European Parliament, Mar 4, 2024.
- “First green light to new bill on firms’ impact on human rights and environment”. Press Release, European Parliament, Mar 19, 2024.
- “From Regulated Prices to Prices Set in Tenders. Tendering landscape in Europe.” White Paper, IQVIA, Accessed Mar 2024.
- “ESG criteria in tendering landscape shows wide variation between top-5 European countries”. Pharmaceutical Technology, Dec, 2020.
- Baltruksa D, Sowab M, Vossa M, “Strengthening sustainability in the pharmaceutical sector”. Centre for Planetary Health Policy, Mar 9, 2023.
- “Evergreen Sustainable Supplier Assessment”. UK NHS, accessed Apr 2024.
- Rauland M, “Sustainability & Pharma Procurement in Europe: Are You ‘Green’ Enough?”. Pharma Boardroom, Nov 11, 2021.
- “Procuring for greener pharma”. Report, Health Care Without Harm, Dec 2022.
- Conic F, Hosseini M, Buente M, “Will environmental sustainability hold sway Over the pharmaceuticals industry?”. Report, Roland Berger, Nov 2022.
- “Sustainability in the pharmaceutical industry”. Association of the British Pharmaceutical Industry, Accessed Mar 2024.
- Agrawal G et al, “Preclinical development of new medicines”. McKinsey & Company, Feb, 2023.

