To Issue 162
Citation: De Silva K, “Closing the Loop: The Latest on Artificial Pancreas Systems”. ONdrugDelivery, Issue 162 (Jun 2024), pp 32–36.
Kamaal de Silva looks at the future of closed-loop insulin delivery systems and how the management of Type 1 diabetes is developing in the digital era.
Closed-loop insulin delivery systems, also known as artificial pancreas systems, are currently in an exciting phase of technological innovation and transformation. These innovations are being driven by enhancements in sensing and delivery technology, promising clinical trial results and new regulatory approvals.
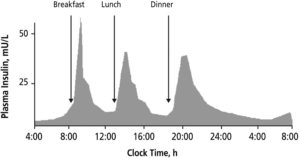
Figure 1: Insulin levels in the blood of a healthy human, showing the sensitivity required to maintain a healthy blood sugar level over the course of the day.2
The development and provision of these systems is currently focused on patients with Type 1 diabetes (T1D). The immune system of these patients attacks the β cells in the pancreas, which create insulin, a hormone that enables the transport of glucose from the bloodstream to the rest of the body. Most of the insulin produced by a healthy β cell is in response to the rising levels of glucose in the bloodstream at mealtimes (Figure 1). Some insulin is also produced by the body during chewing in preparation for a blood sugar spike.1
Patients with T1D must inject insulin to manage their blood glucose levels over the course of the day. With a manual system, calculation of the required insulin dose can be a complex process, requiring patients to constantly monitor glucose levels and use that information to plan and execute calculations at specific times.3 The effect of a certain dose of insulin even has its own day-to-day variability, which is difficult to predict. Most patients with T1D are therefore unable to achieve their recommended glycaemic targets.4
“Artificial pancreas systems aim to bring management of T1D into the digital era.”
Artificial pancreas systems aim to bring management of T1D into the digital era. The essential components are a continuous glucose monitor (CGM), an insulin pump and a control algorithm (Figure 2). Most systems are known as “hybrid”, meaning that even though the insulin rate is adjusted automatically, users still have some interaction with the system, for example, counting and entering mealtime carbohydrates.5
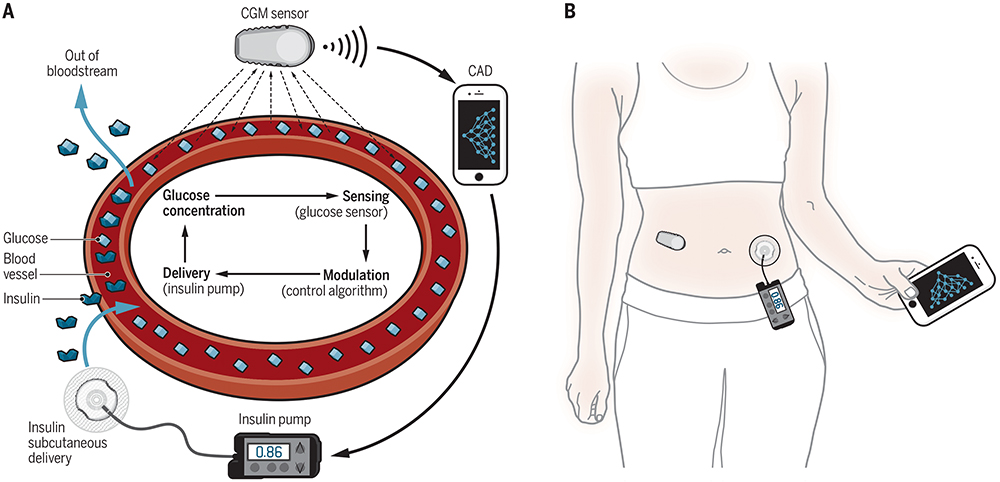
Figure 2: The key components of an artificial pancreas system.6
“The impressive accuracy of current monitors is the result of year-on-year performance improvements.”
A SPIKE IN PRECISION
CGMs measure glucose levels in the interstitial fluid between cells in the abdomen or back of the upper arm, normally via an enzymatic reaction. There is an inherent delay of approximately10 minutes7 in the reading when measuring blood glucose in this way, which affects the accuracy of the reading and therefore the performance of the control algorithm.
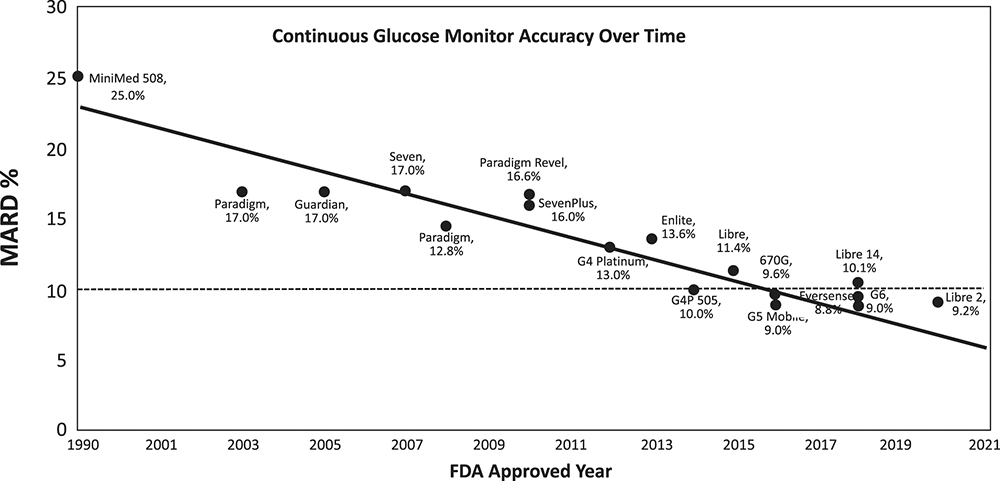
Figure 3: Improvements in CGM MARD since 1990.9
As shown in Table 1, the newest CGMs can achieve a mean absolute relative difference (MARD) of 8% when compared with a calibrated reference, report glucose levels every minute and do not require user calibration with a fingerstick reading. The impressive accuracy of current monitors is the result of year-on-year performance improvements, as can be seen in Figure 3, where a MARD of <10% is considered accurate enough for insulin dose calculations.8 It is currently unclear whether the accuracy of new sensors will continue to improve or begin to flatten out as we approach the limit of the technology.
| FDA approval (year) |
CE mark (year) | MARD (%) |
Warm-up time (hours) |
Service life (days) |
|
| Abbott FreeStyle Libre 3 | 2022 | 2022 | 7.9 | 1 | 14 |
| Dexcom G7 | 2022 | 2020 | 8.2 | 0.5 | 10 |
Table 1: Technical details of the most recently approved CGMs.
Further research in this area is ongoing to ensure that the accuracy of the CGM is maintained over a range of use cases. For example, compression of the device when sleeping and long periods of aerobic exercise have been shown to produce erroneous readings. There are also efforts to reduce the effects of various interfering substances, such as ascorbic acid, hydroxyurea and others yet to be identified.10
SMART PUMPS AND APPS
The control algorithm can either be integrated into the pump itself or located on a separate device, which can even be a smartphone. The safety of the control system is paramount, as delivering too much insulin can cause hypoglycaemia, which can quickly become life threatening if untreated.
The three main types of control algorithms used in approved systems are:
- Proportional-integral-derivative (PID): One of the most common control methods, a predefined PID equation, uses an error value in parallel to make corrections. A pump using this algorithm is therefore more dependent on live blood sugar readings to make frequent adjustments. The current level of insulin in blood plasma has a suppressive effect on the amount of new insulin secreted by healthy ß cells, and this effect is too quick for a PID system to mimic using interstitial blood sugar readings from a CGM. PID algorithms usually incorporate an insulin feedback (IFB) algorithm to account for this.11
- Fuzzy logic (FL): Used in Medtronic’s (MN, US) new MiniMed 780G in conjunction with PID control, FL mimics human vagueness in decision making when working with incomplete data. This improves the algorithm’s response to unexpected situations, such as illness, exercise or unplanned meals.12
- Model predictive control (MPC): Generally regarded as a more advanced method of control, where new predictive models over a defined “horizon” are rapidly generated and updated as blood sugar levels change. MPC is therefore considered preferable for artificial pancreas systems that have large time delays or predictable dynamics. However, as with PID, the algorithm may require additional controls to deal with disturbances that break from the models.
Most of the artificial pancreas systems in Table 2 are now approved in both the EU and US. Medtronic, the first entry into the market, operates within a closed ecosystem, whereas newer entries by competitors typically use Dexcom (CA, US) and/or Abbott (IL, US) CGMs. This potentially gives users more choice and reduces any possibility of geographical supply limitations. However, Medtronic’s ownership of the whole ecosystem could allow it to be closer to the sensor technology and implement improvements in sensor technology directly into their own algorithm.
| Pump | FDA approval (year) | CE mark (year) |
Algorithm | CGM compatibility | |
| Medtronic | MiniMed 670G | 2016 | 2018 | PID with IFB | Medtronic Guardian 3 |
| MiniMed 770G | 2020 | PID with IFB | Medtronic Guardian 3 | ||
| MiniMed 780G | 2023 | 2020 | PID with IFB & FL | Medtronic Guardian 4 | |
| Tandem | t:slim X2 with Control-IQ | 2022 | 2018 | MPC | Dexcom G6/G7 |
| Insulet | Omnipod | 2022 | 2024 | MPC | Abbott FreeStyle Libre 3 Dexcom G6 |
| BetaBionics | iLet Bionic Pancreas | 2023 | MPC | Dexcom G6/G7 | |
Table 2: A summary of approved hybrid closed-loop systems for T1D in Europe & the US where the control algorithm is on the pump.
With the systems shown in Table 3, placing the controller on a separate device increases the potential for device interoperability, but there are other trade-offs. In the case of Diabeloop’s (Grenoble, France) DBLG1, the user must carry another handheld device with them. As an Android/iOS app, CamDiab’s (Cambridge, UK) CamAPS FX does not encounter this problem for most users, but could leave diabetes management vulnerable to typical problems with apps, such as issues arising when using specific smartphones and OS versions.
| Description | CE mark (year) | Algorithm | Pump compatibility | CGM compatibility | |
| Diabeloop DBLG1 | Purpose-built touchscreen handset using Android | 2018 | MPC | ViCentra Kaleido Roche Accu-Chek Insight |
Dexcom G6 |
| CamDiab CamAPS FX | App available for Android and iOS | 2022 | MPC | Ypsomed mylife YpsoPump Advanced Therapeutics DANA Diabecare RS and DANA-i |
Abbott FreeStyle Libre 3 Dexcom G6 |
Table 3: A summary of approved hybrid closed-loop systems for T1D, currently only approved in Europe, where the control algorithm is on a separate device.
“There are currently thousands of people around the world using some form of DIY closed-loop system.”
#WEARENOTWAITING
There are currently thousands of people around the world using some form of DIY closed-loop system.13 The movement has centred around the hashtag #WeAreNotWaiting, with the intention of improving the availability and affordability of artificial pancreas systems. For example, in the US a new insulin pump and transmitter can cost up to US$7,400 (£5,815), not including $420 for a 30-day supply of sensors.14 In 2023 it was announced that hybrid closed-loop systems would be made available for UK NHS patients over the next five years.15
The three main DIY artificial pancreas systems in use today are:
- OpenAPS: The first system to be developed in 2016 using a Raspberry Pi to control a set of now unsupported Medtronic pumps released between 2003 and 2010 (Figure 4)
- Loop: A similar approach but designed to work on an iPhone. It is compatible with the same Medtronic pumps as OpenAPS with the addition of the Omnipod
- AndroidAPS: Which has the same core functionality of OpenAPS but can be installed on an Android smartphone.

Figure 4: A DIY artificial pancreas system from 2016, using a battery-powered Raspberry Pi, Dexcom receiver, and CareLink USB stick transmitting to a Medtronic pump.16
Interestingly, these systems are not based on any of the aforementioned advanced control algorithms, but rather designed to emulate the user’s manual decision-making process when using a bolus pump. The systems can also be configured to provide the exact amount of automation and responsiveness needed for a specific user. Some studies have reported similar performance for key metrics, such as time in range and HbA1c levels, when compared with approved hybrid closed-loop systems.17 However, creating a working DIY system requires some technical know-how.
In 2023, a diabetes non-profit charity, Tidepool (CA, US), received clearance from the US FDA to proceed with Tidepool Loop. This is an effort to bring an easier to use but open-source system into regulatory control. Unfortunately, the system is currently not available for use as it requires pump manufacturers to gain FDA clearance for their use with an alternative controller.18
A BIONIC FUTURE
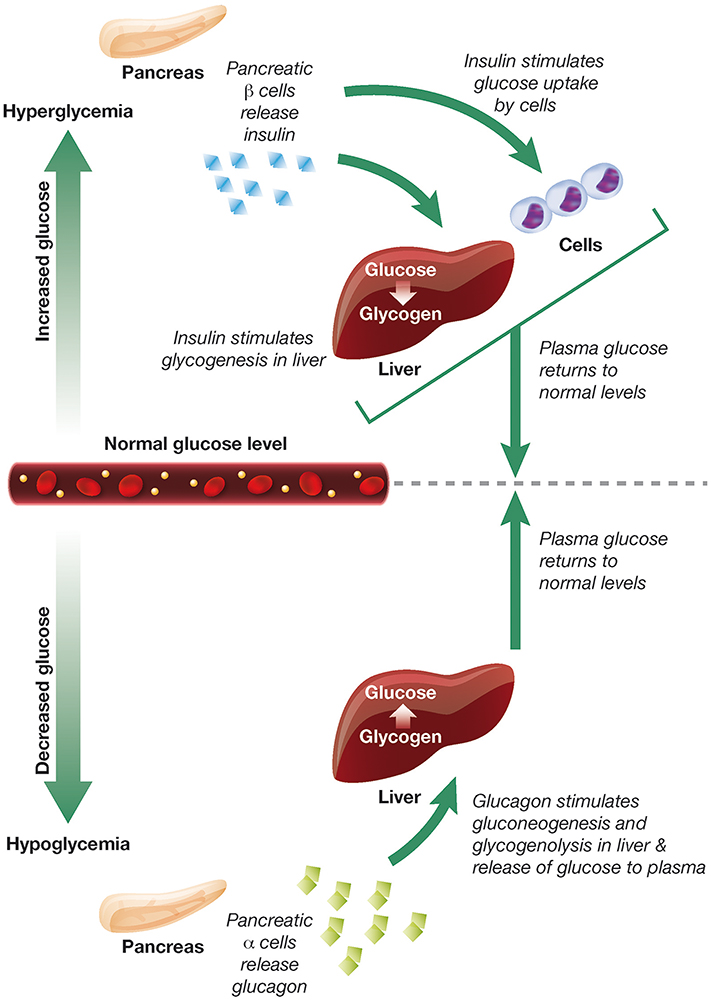
Figure 5: The opposing roles of insulin and glucagon in the regulation of blood glucose levels.20
The artificial pancreas systems of the future could develop in several ways. Control algorithms could improve, helping to overcome the time delay in sensing blood sugar levels, as well as the absorption and action of insulin. Machine learning and artificial intelligence (AI) could play a key role in generating bespoke predictive models. Also, the introduction of ultra-rapid-acting insulin analogues, such as Fiasp (Novo Nordisk) and Lyumjev (Eli Lilly), could increase system responsiveness.19
Patients with T1D are also deficient in the production of other hormones that control blood sugar, such as glucagon. Glucagon increases blood sugar levels from stores, so could allow for more aggressive insulin dosage during spikes while reducing the risk of hypoglycaemia (Figure 5). Subtle pancreatic regulators, such as amylin analogues (pramlintide) and GLP-1 agonists, could also be added to the mix. Every new development in the artificial pancreas story has been an exciting step forwards, and it is clear that this trend will continue long into the future.
REFERENCES
- Eliasson B et al, “Cephalic phase of insulin secretion in response to a meal is unrelated to family history of type 2 diabetes”. PLoS One, 2017, Vol 12(3), Article e0173654.
- Thompson R, Christie D, Hindmarsh PC, “The role for insulin analogues in diabetes care”. Current Paediatrics, 2006, Vol 16(2), pp 117–122.
- Vloemans AF et al, “Youth With Type 1 Diabetes Taking Responsibility for Self-Management: The Importance of Executive Functioning in Achieving Glycemic Control”. Diabetes Care, 2019, Vol 42(2), pp 225–231.
- Davies M, “The reality of glycaemic control in insulin treated diabetes: defining the clinical challenges”. Int J Obes, 2004, Vol 28(S2), pp S14–S22.
- “Story of Discovery—Artificial Pancreas for Managing Type 1 Diabetes: Cutting-edge Technology 50 Years in the Making – NIDDK”. National Institute of Diabetes and Digestive and Kidney Diseases, Jan 2017.
- Boughton CK, Hovorka R, “Advances in artificial pancreas systems”. Sci Transl Med, 2019, Vol 11(484), Article eaaw4949.
- Schmelzeisen-Redeker G et al, “Time Delay of CGM Sensors: Relevance, Causes, and Countermeasures”. J Diabetes Sci Technol, 2015, Vol 9(5), pp 1006–1015.
- Kovatchev BP et al, “Assessing Sensor Accuracy for Non-Adjunct Use of Continuous Glucose Monitoring”. Diabetes Technol Ther, 2015, Vol 17(3), pp 177–186.
- Bailey TS, Alva S, “Landscape of Continuous Glucose Monitoring (CGM) and Integrated CGM: Accuracy Considerations”. Diabetes Technol Ther, 2021, Vol 23(S3), p S-5.
- Friedman JG et al, “Use of Continuous Glucose Monitors to Manage Type 1 Diabetes Mellitus: Progress, Challenges, and Recommendations”. Pharmgenomics Pers Med, 2023, Vol 16, pp 263–276.
- Ruiz JL et al, “Effect of Insulin Feedback on Closed-Loop Glucose Control: A Crossover Study”. J Diabetes Sci Technol, 2012, Vol 6(5), pp 1123–1130.
- Mauseth R et al, “Use of a ‘fuzzy logic’ controller in a closed-loop artificial pancreas”. Diabetes Technol Ther, 2013, Vol 15(8), pp 628–633.
- “OpenAPS Outcomes”. Web Page, OpenAPS, accessed May 2024.
- Dermawan D, Kenichi Purbayanto MA, “An overview of advancements in closed-loop artificial pancreas system”. Heliyon, 2022, Vol 8(11), Article e11648.
- “New ‘artificial pancreas’ technology set to change the lives of people having difficulty managing their type 1 diabetes”. NICE, Jan 2023.
- Williamson-Lee J, “Diabetes patients’ DIY solutions are still the standard of care”. Mashable, October 2021.
- Kesavadev J et al, “The Do-It- Yourself Artificial Pancreas: A Comprehensive Review”. Diabetes Ther, 2020, Vol 11(6), pp 1217–1235.
- Downey L et al, “A European regulatory pathway for Tidepool loop following clearance in the United States?”. Diabet Med, 2024, Vol 41(4), Article e15246.
- Hartnell S et al, “Closed-loop technology: a practical guide”. Practical Diabetes, 2021, Vol 38(4), pp 33–39.
- Haedersdal S et al, “The Role of Glucagon in the Pathophysiology and Treatment of Type 2 Diabetes”. Mayo Clin Proc, 2018, Vol 93(2), pp 217–239.

