To Issue 168
Citation: Latham D, Allen M, Dean C, “Next-Generation Autoinjectors: Balancing Patients, Pharma, Payers and Planet”. ONdrugDelivery, Issue 168 (Jan 2025), pp 32–36.
David Latham, Matthew Allen and Charlie Dean use a case study of a reusable electromechanical autoinjector to outline the novel product development approach needed to achieve better outcomes for patients, pharma, payers and the planet.
The stakeholder needs in drug delivery device development are continually evolving, resulting in an increasing breadth of variables at play and the number of tools required. Having a clear product development roadmap created with iterative design inputs from multiple disciplines is key to determining the optimal path to satisfy these conflicting requirements. This succeeds by elevating conventional product development with the integration of business strategy, product cost, environmental product stewardship and patient-centric design. To unlock this potential, a culture of smart risk-taking is also necessary. Discovering dead ends early on and tackling the highest risk areas first gives the best development value to investment ratio.
“The prevalence of biologics to treat chronic diseases means that the number of patients injecting themselves at home has grown significantly over the past few decades.”
WHAT IS MISSING WITH CURRENT AUTOINJECTOR PLATFORMS?
The introduction of autoinjector drug delivery systems in the 1970s significantly improved the standards of care by removing the need for multiple healthcare professional (HCP) consultations and allowing patients to treat themselves. The prevalence of biologics to treat chronic diseases means that the number of patients injecting themselves at home has grown significantly over the past few decades.
Evolution of the core drug delivery technology has, however, been limited. The most common devices are single-use, disposable mechanical systems with a prefilled syringe (PFS) and staked needle. Device activation is either two-step contact-activated or three-step button-activated systems. Sharps injury protection features appeared around the turn of the millennium and have become an expected, if not required, feature. The product portfolio has extended beyond 1 mL PFS platforms, to 2.25 mL, and is now pushing towards the 5 mL mark, where higher-viscosity formulations and higher-gauge needles require greater spring force. However, features such as variable injection depth setting, variable injection speed control and connectivity have remained niche improvements.
“Recent developments have seen reusable devices that show potential to reduce therapy cost and environmental burden but have drawbacks that limit their widespread adoption.”
Recent developments have seen reusable devices that show potential to reduce therapy cost and environmental burden but have drawbacks that limit their widespread adoption. Sharp injury protection is either omitted or achieved using a cassette sub-assembly attached to the PFS by the manufacturer or by the use of a PFS with passive needle retraction at the end of dose. Mechanical, spring-driven systems that are manually reset by the patient introduce usability and plunger force challenges.
Electromechanical devices have had limited appeal largely due to their high unit costs of incorporating electronic elements and the temptation to make feature-rich concepts where the business case does not justify the extra feature(s) inclusion.
Looking into the broader needs and desires of patients, pharma, payers and the planet (the 4Ps), there are conflicting requirements (Table 1).1 Pharma and medical device organisations need to innovate to redress the 4Ps imbalance.
| Patients | Availability, ease of use, safety and reliability, convenience, minimal pain. |
| Pharma | Grow sales and profit in both the short and long term. Minimise capital expenditure and internal investment.
|
| Payers | Minimising cost burden of treatment. Affordable co-pay in applicable markets. Stay within agreed budgets, often for fixed accounting periods (e.g. annual). |
| Planet | Reducing emissions and waste associated with therapy. Maximising resource efficiency and moving towards the circular economy. |
Table 1: Summary of needs for the 4Ps.
There is a need for new autoinjector technology that is based on PFS primary packaging, which allows users to self-administer with the same or improved level of safety and efficacy while making trade-offs in other areas to achieve economic and environmental goals. A development environment is needed to enable product development teams to look again at the real challenges and develop new devices that are unconstrained by current technology.
CALL TO ACTION
The mission was to demonstrate how a new autoinjector design might answer the aforementioned challenges: maintaining the levels of safety and efficacy provided by current disposable mechanical autoinjectors while reducing both financial and environmental costs. The requirements are:
- Must use ISO-standard PFS containers currently used on validated filling lines
– Avoiding any new investment in filling systems and use economies of scale of existing PFS components
– Including 1 and 2.25 mL sizes, with scalability to 5 mL and beyond, both cut flange and small round flange - Must support a range of fill volumes and a range of viscosities greater than those commonly seen today in autoinjectors.
“The design vision was controlled by a simple hierarchy of optimisation.”
The design vision was controlled by a simple hierarchy of optimisation (Figure 1). Working on the same principle as Maslow’s hierarchy of needs, the base layer was satisfying all core autoinjector functionality and being able to pass verification and validation. The core functions were based on current disposable mechanical device core features, with the option to add or remove technology-dependent features later if relevant. Once this layer was satisfied, the second layer of cost reduction was targeted.
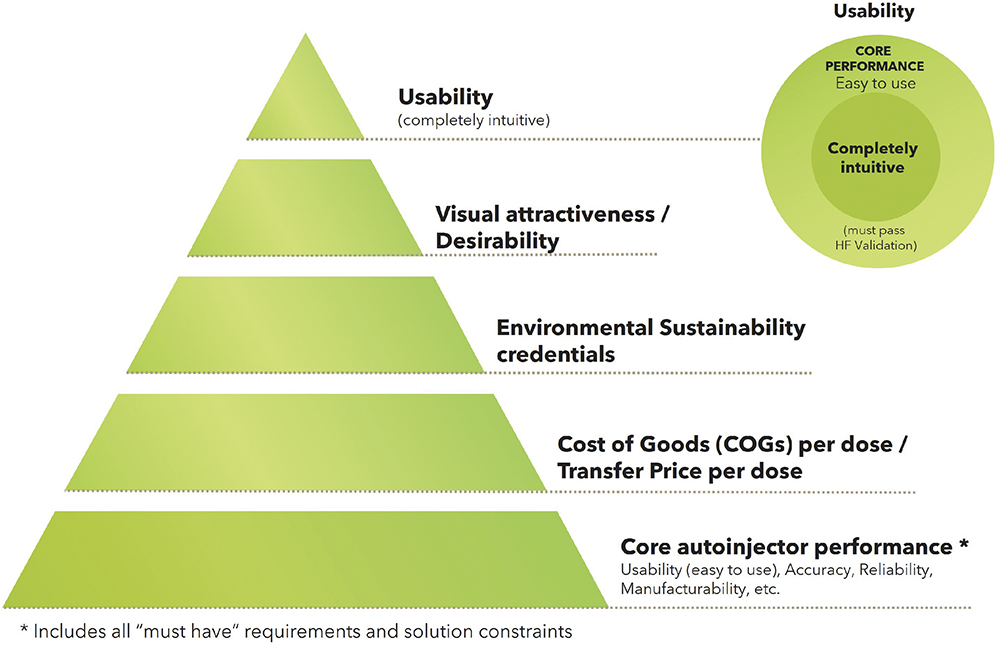
Figure 1: Hierarchy of optimisation.
Cost was second because a compelling business case to stakeholders must be demonstrated. Cost optimisation also has synergies with the third layer of environmental sustainability and could be reversed in some cases.
The fourth layer was desirability. Its inclusion was a clear acknowledgement of the importance of good design to multiple stakeholders, not least the commercial marketing teams.
The fifth and final layer was enhanced usability, providing an emphasis to seize any remaining opportunities to go beyond easy to use and move towards completely intuitive to use.
A VISION OF WHAT IS POSSIBLE
What resulted from this project were two reusable electromechanical autoinjector variants (Figures 2 and 3). In both variants, the only disposable part is the naked PFS components with no add-on cassette. The reusable device provides sharps injury prevention, needle hiding and usability through mechatronics, which include automatic removal and replacement of the needle cap and the detection of premature lift. The impact of including limited electronics in the reusable unit was more than outweighed by the savings brought by not having a cassette on the disposable part, even before considering savings in shipping and storage costs.

Figure 2: Bamboo variant 1, three-step, contact-activated electromechanical reusable autoinjector.
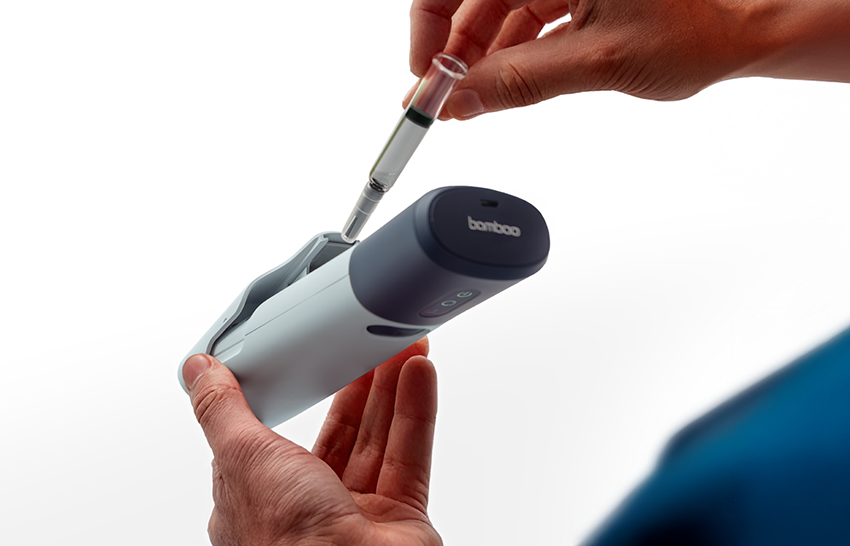
Figure 3: Bamboo variant 2, four-step, button-activated electromechanical reusable autoinjector.
While purely mechanical concepts that achieved the same functionality were investigated, it became apparent that they would be unlikely to fulfil the “easy to use” requirement. This type of system would also introduce significant mechanism complexity overall and greatly limit the potential plunger force, thus reducing the suitability for high-volume and/or high-viscosity drug products. On the commercial side, removing the need for a cassette also keeps the business model simple – avoiding proprietary “printer cartridge style” models that can lead to higher lifetime costs as the single-use element is sold at a higher mark-up to discount the price of the reusable device.
The other key factor for reducing costs and increasing sustainability was identifying the relative contributing factors in the device. Motors are by far the single biggest financial cost contributor to the bill of materials, while printed circuit boards and motors have the largest impact by mass on environmental sustainability. Therefore, given that the device essentially has three groups of automated mechanical functions – needle cap removal and replacement, needle insertion and retraction, and dose delivery – the key was using either one or two motors to deliver these functions. The architecture devised only required a single motor and minimal printed circuit board size to deliver all the automated mechanical functions. In parallel, high-quality non-medical-grade motors were identified and it was confirmed that they delivered the required performance when combined with low-cost controls – all at a fraction of the cost of traditional medical-grade motors.
Combining the automatic uncapping and recapping of the needle shield with an architecture that has a single motor performing multiple automated functions leads to the financial and environmental cost per dose figures shown in Figure 4 (excluding the impact of drug product and filling but including other PFS components), which have been benchmarked against a current industry standard disposable mechanical autoinjector assessed using the same methodologies.
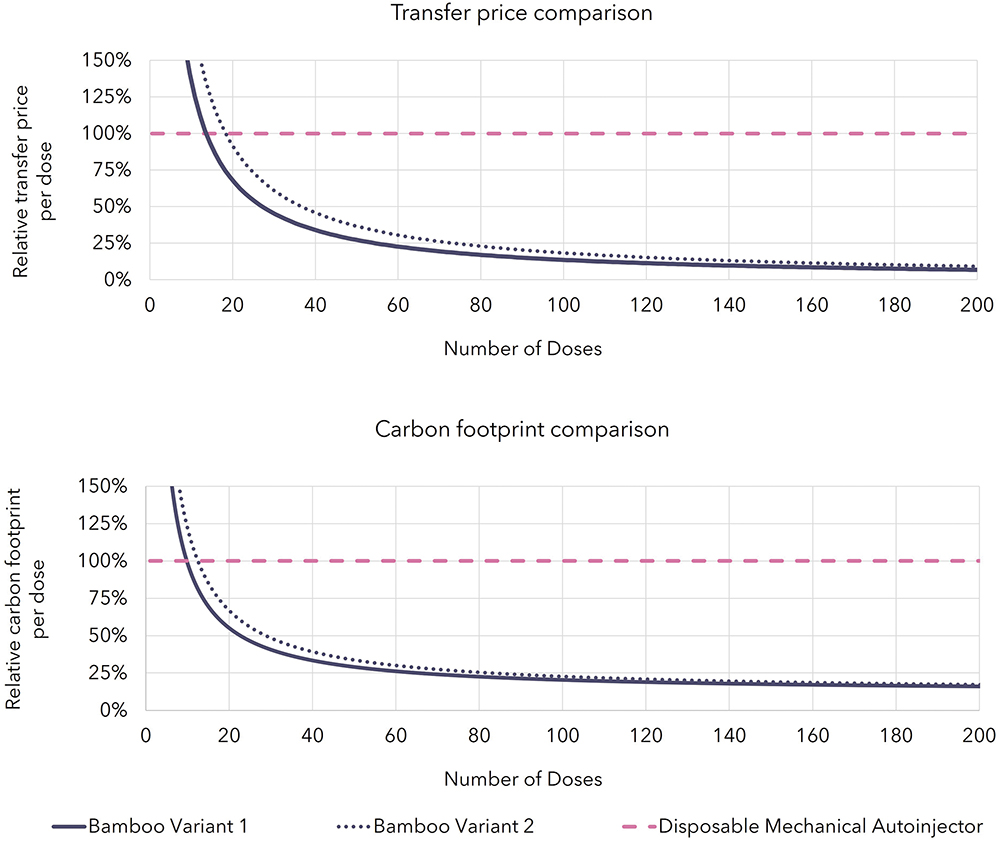
Figure 4: Transfer price (top) and carbon footprint (bottom) comparison of autoinjector technologies.
The figures show the impact per dose asymptote to the impact of the PFS components for both reusable electromechanical autoinjector variants. More importantly, they show the financial cost break-even point with mechanical disposable products for cost occurring after just 14–18 doses even when ignoring the extra savings associated with shipping and storage. For carbon footprint, the break-even point comes sooner at 10–13 doses. These figures make a compelling case for the reusable autoinjectors’ viability for PFS-based products that have dosing frequencies down to once monthly or less frequently, with the business case looking even more attractive as the dosing frequency increases.
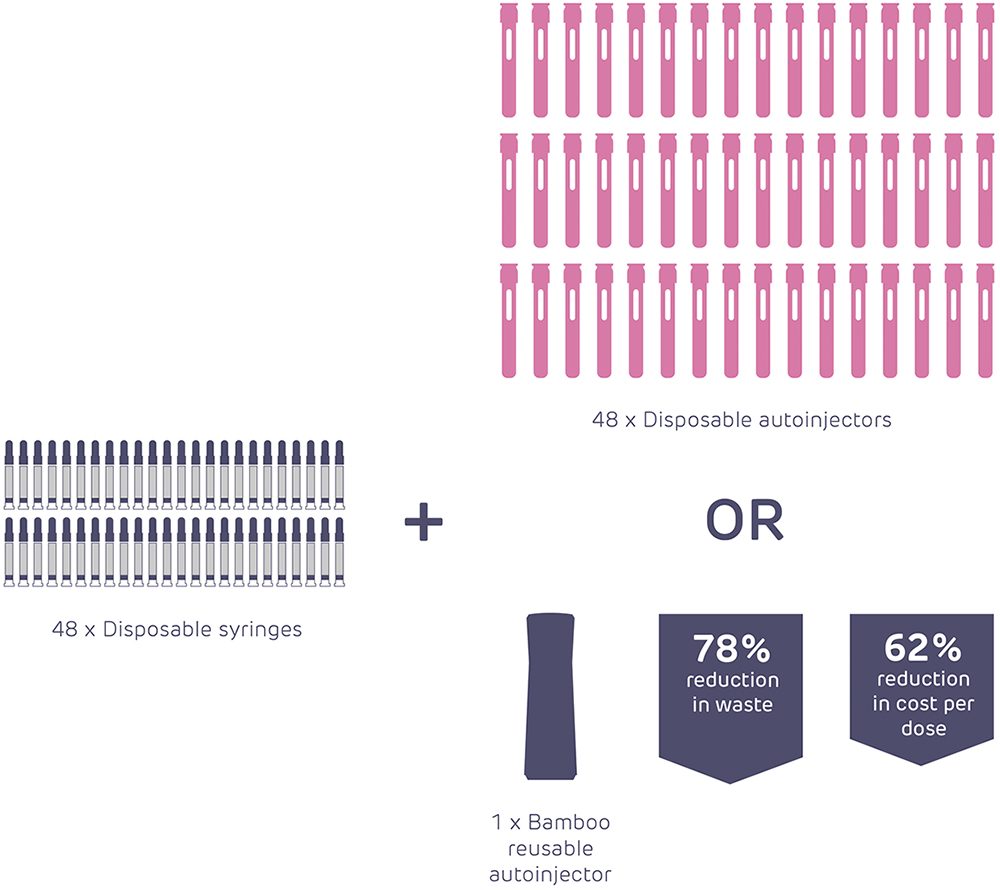
Figure 5: Illustrative comparison of material usage between disposable and reusable autoinjectors.
“By applying the methodologies and capabilities that are needed to address the challenges listed at the start of this article, we can see there is enormous scope to improve the current state of the art even without any quantum leaps in technology.”
By applying the methodologies and capabilities that are needed to address the challenges listed at the start of this article, it can be seen that there is enormous scope to improve the current state of the art even without any quantum leaps in technology (Figure 5).
The electromechanical architecture has allowed advances in usability and desirability:2 true end-of-dose user feedback or “safe to lift from injection site” feedback to reduce the risk of wet injections; consistent injection time; the absence of stored mechanical energy at the end of injection; and avoidance of loud or irregular noises during use. In addition, they enable delivery of drug viscosities far outside the range of standard mechanical autoinjectors – and delivery of standard viscosities through smaller diameter needles – due to the significantly higher plunger rod stall forces (170 N in the autoinjector example).
In conclusion, current disposable mechanical autoinjectors have served the market extremely well over the past few decades and will continue to play a role going forwards. However, for the current and future needs of the 4Ps in treating chronic diseases safely and effectively at home, autoinjector technology needs to evolve to reduce both the financial and environmental burden on healthcare systems and the planet. Fortunately, this case study has demonstrated that there is ample scope to dramatically improve both. This can be achieved without compromising the current levels of clinical safety and efficacy (and possibly improving it in some respects), with the proviso that compromises on other elements, such as convenience, are accepted. Whether this is compelling enough to effect change among key decision makers remains to be seen; to make the leap of faith in pursuing a fresh paradigm of home-use parenteral drug delivery over the “faster horse” option of selecting established autoinjector types.
ACKNOWLEDGEMENTS
Scott Lewis – Senior Consultant, Mechanical Design and Manufacturing, Healthcare, Cambridge Consultants; John Sendall – Principal Engineer, Mechanical Design and Manufacturing, Healthcare, Cambridge Consultants; Christopher Harm – Senior Industrial Designer, Design, Healthcare, Cambridge Consultants.
REFERENCES
- Whelton R, Green P, “Autoinjectors: Historical Achievements and Compelling Needs Driving Next-Generation Devices”. ONdrugDelivery, Issue 138 (Oct 2022), pp 14–17.
- Sørensen B, “Re-usable Electronic Autoinjector – Flexible Performance”. ONdrugDelivery, Issue 120 (May 2021), pp 44–46.

