Citation: Barichello E, Prete R, “Protection and Production: Optimising Primary Packaging Solutions for mRNA Therapies”. ONdrugDelivery, Issue 172 (May 2025), pp 90–95.
Enrico Barichello and Riccardo Prete discuss the impact that mRNA-based therapies are having on the modern pharmaceutical market and how these delicate therapies require careful handling, with a particular focus on how Stevanato Group’s portfolio of primary packaging products can be highly beneficial when bringing these critical medicines to market.
Over the past five years, significant advances have been made across the pharmaceutical sector, particularly with the rise of messenger RNA (mRNA) therapies. This development was propelled by the successful creation of the world’s first mRNA-based vaccine, which demonstrated the potential of this technology. This was a milestone achievement that underlined the promise of mRNA technologies and fuelled further advances in their use to tackle a wider range of diseases, including cancer and rare genetic conditions, beyond their continued development as a potent platform for vaccines.
“THERE ARE MORE THAN 450 mRNA DRUGS AT THE PRECLINICAL AND CLINICAL TRIAL STAGE, WITH mRNA PROVING TO BE THE PREFERRED RNA MODALITY IN THE RESEARCH PIPELINE.”
In mRNA therapies, molecules that are inherently prone to degradation are transported into cells within the protective wrapper of a carrier or vector, such as a lipid nanoparticle (LNP).1 Safely at its target site, the mRNA delivers its “message”, instructing the body to produce protective proteins that bolster immunity and safeguard against the effect of disease. Boosted by the catalyst of covid-19, mRNA vaccines are expected to register a compound annual growth rate (CAGR) of 18% in the years leading up to 2030, significantly outstripping the 4% growth expected for traditional vaccines over the same period, according to IQVIA data. More widely, it is estimated that there are more than 450 mRNA drugs at the preclinical and clinical trial stage, with mRNA proving to be the preferred RNA modality in the research pipeline.2
PRIMARY PACKAGING FOR mRNA THERAPIES
For the innovators behind these developments, the challenge of bringing an effective mRNA therapy to market is not limited to the complexities of formulation science. Indeed, the choice of primary packaging is also a crucial concern, and there are various important considerations to incorporate into this decision-making process.
“ARGUABLY, THE PRIMARY QUESTION IS HOW TO ENSURE THAT THE STABILITY OF THE mRNA THERAPY IS SUPPORTED OVER THE PRODUCT LIFECYCLE, AS THIS HAS CRITICAL BEARING ON ITS POTENCY AND EFFICACY WHEN A PATIENT IS INJECTED.”
Arguably, the primary question is how to ensure that the stability of the mRNA therapy is supported over the product lifecycle, as this has critical bearing on its potency and efficacy when a patient is injected. Various influences are known to have a negative impact on stability, including ribonuclease enzymes, excipients and pH levels.3 The presence of particulates in the container closure system also introduces potential for degradation. Moreover, it is essential to safeguard sterility and container closure integrity (CCI), as well as to ensure that there is no interaction with contact surfaces that might compromise the drug product quality. These characteristics underline the importance of containing mRNA therapies within primary packaging components that can accommodate their physicochemical fragility.
Another factor that has a major bearing on stability in mRNA therapies is temperature. Indeed, the first mRNA covid-19 vaccines placed significant demands on logistics through their ultra-cold storage requirements. While advances in formulation science continue to address the difficulties associated with maintaining low-temperature conditions throughout manufacture, distribution and storage, the need to maintain CCI within deep-cold storage conditions remains a priority for mRNA therapies.4
All of this highlights the importance of addressing the physical, chemical and thermal risks associated with primary packaging. Particularly in mRNA applications, it is also important to note some of the pragmatic considerations in relation to manufacture and fill-finish. With high value and small batch sizes, close control must be maintained over all stages of production. Enforcing the highest levels of quality and being able to rely on the highest quality primary packaging components ensures that the risk of drug wastage is minimised and production efficiency is optimised.
This is particularly the case for vaccines, where the rapid evolution of a virus can precipitate equally rapid development of updated versions in order to keep populations protected. These new iterations must, of course, be packaged appropriately, whether in vials or prefilled syringes (PFS), placing a higher burden on production facilities to be flexible while limiting impact on time and cost. In this context, the benefit of accessing the right containment solution is significantly augmented by the flexibility to quickly and easily adapt fill-finish environments, keeping any production changes down to an absolute minimum.
THE EZ-FILL® PLATFORM
Working with Stevanato Group, companies developing mRNA therapies have access to a comprehensive portfolio of containment solutions that integrates a robust supply of high-quality products with extensive manufacturing expertise to simplify the complex issues that must be considered for the packaging of mRNA therapies. Stevanato Group’s EZ-fill® customisable containment solution, for example, presents vials, syringes and cartridges in a ready-to-use (RTU) format to streamline aseptic fill-finish processing, reducing both time-to-market and total cost of ownership. Available in nest-and-tub and tray configurations, EZ-fill containers are separated in the secondary packaging to prevent the risk of contact and damage during transportation, and at buffering or in-feeding points in production. This limits glass-to-glass interaction – a key source of breakages, cosmetic issues and particle creation – to maintain container integrity and limit rejects.
“FOR ALL COMPONENTS, MANUFACTURE AND SUPPLY OF THE PRIMARY CONTAINER HAS BEEN OPTIMISED TO INCREASE REGULATORY ACCEPTANCE AND SUSTAINABILITY, FURTHER REDUCING TOTAL COST OF OWNERSHIP.”
EZ-fill has been further enhanced by Stevanato Group’s EZ-fill Smart® platform. Here, for all components, manufacture and supply of the primary container has been optimised to increase regulatory acceptance and sustainability, further reducing total cost ownership. With no glass-to-glass and minimised glass-to-metal contact throughout a highly automated production process, as well as the option of low-temperature vaporised hydrogen peroxide sterilisation and the inclusion of secondary packaging with lower plastic content, EZ-fill Smart provides all the elements required to satisfy the demands of a rigorous particle-reduction strategy.
As with all RTU containment solutions, because Stevanato Group manages all the preliminary stages of packaging, washing and sterilisation, the burden on pharmaceutical companies is reduced, with fewer operational processes required and less demand on cleanroom space. Furthermore, the company’s comprehensive testing for processability with leading equipment suppliers ensures that containers can be seamlessly integrated into the fill-finish workflow. For applications where mRNA therapies are best suited to delivery via a PFS − with their advantages of dose containment, delivery convenience and waste reduction − Stevanato Group’s containment portfolio offers the same level of support for CCI, drug stability and production flexibility as for vial-based solutions.
ALBA® GLASS SYRINGES
The Alba® range of glass-based syringes has been designed specifically for sensitive drug formulations where there is a heightened risk of interaction between the product and the container closure system. Rather than using a conventional coating, Alba features an innovative cross-linked silicone coating technology, providing enhanced protection against silicone oil particles, interactions between the drug and the container surface, extractables and pH shift, all without compromising the glide and break-loose performance (Figure 1).
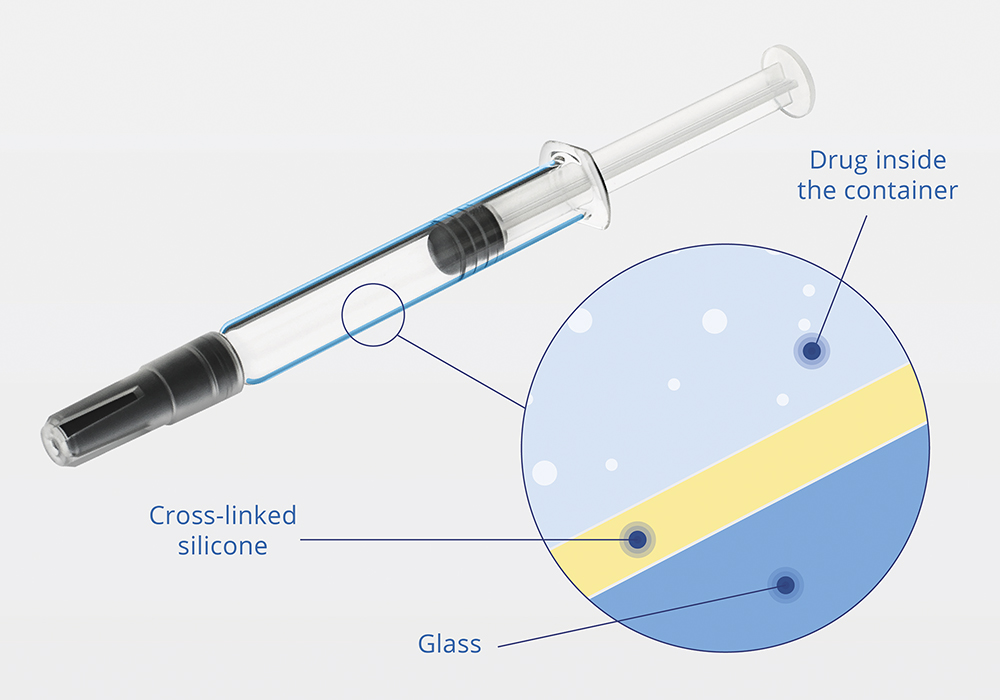
Figure 1: Cross-linked silicone coating in an Alba® syringe.
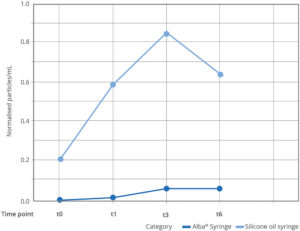
Figure 2: Results of subvisible particle (≥10 μm) testing at -40°C over six months. Alba’s data at the 12-month time point are consistent with the findings from the preceding checkpoint.
The significant reduction in silicone particle migration with Alba syringes has been demonstrated through extensive in-house testing. These tests compared Alba against syringes featuring traditional silicone oil coating under storage conditions of -40°C to evaluate the levels of subvisible particles present in a placebo solution over time using light obscuration. The results clearly show the effectiveness of the cross-linked coating technology in keeping silicon particles to an absolute minimum (Figure 2).
Further tests reinforced that the storage conditions resulted in no noticeable impact on break-loose force and extrusion over time for Alba syringes. The same is true for CCI integrity, where Alba has been tested at -70 °C without reporting any failure among the samples tested (Figure 3).
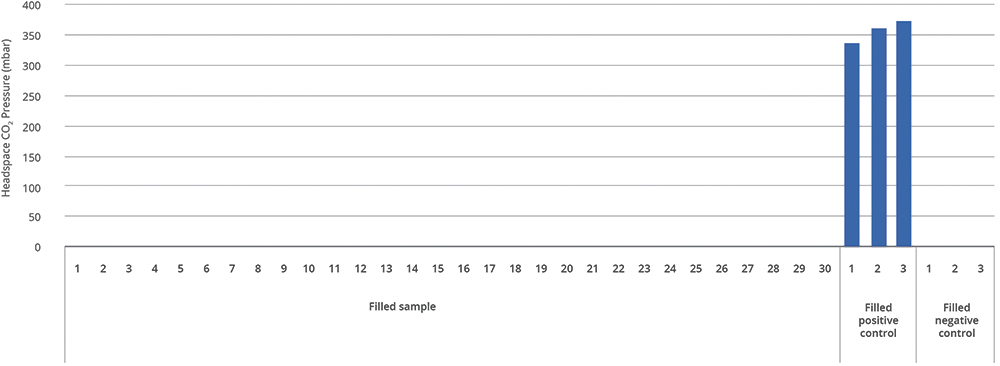
Figure 3: Alba syringe and CCI results for filled 1 mL long syringe at -70°C.
Figure 4: Stevanato Group’s Nexa Flex™ polymer syringe.
NEXA FLEX™ POLYMER SYRINGES
In PFS applications where polymer is preferred over glass, Stevanato Group’s diverse product range extends to the Nexa Flex™ pre-sterilised syringe, which also features a lower silicone particle profile (Figure 4). Available both in cyclic-olefin polymer (COP) and cyclic-olefin copolymer (COC), Nexa Flex containers are manufactured using a tungsten-free moulding process to limit interaction with sensitive drug products, such as mRNA therapies.
Nexa Flex syringes feature a similar coating to Alba syringes, with the polymerisation process incorporating silicone cross-linking to create an advanced protective barrier that reduces the risk of silicon particle creation. Stevanato Group’spartnerships with leading elastomer manufacturers also ensure that CCI is maintained for the syringe in cold storage conditions, with testing demonstrating equivalent levels of shrinkage between the plunger and polymer container to provide assurance that the drug product remains protected (Figure 5).
Figure 5: Evaluation of seal integrity during cold storage at -80°C using 1 mL Nexa Flex syringe with two silicone recipes and three test formulation concentrations (low/medium/high).
AVOIDING DISRUPTIONS TO MANUFACTURING AND FILL-FINISH
While the characteristics demonstrated by Stevanato Group’s primary packaging portfolio are critical for supporting the stability of sensitive mRNA therapies throughout their lifecycle and under potentially demanding conditions, the company has also designed its primary packaging options to provide benefits to manufacturers through the flexibility they offer in fill-finish environments. Here, there is potential for major disruption when adjusting production lines for relatively small batches of mRNA therapies based on the need to change an already validated infrastructure to align with the requirement of a different formulation and containment choice.
However, the flexibility of Alba, offered in the same formats as standard vaccine syringes, including secondary packaging, means that it is possible to introduce this syringe with little to no disruption to current manufacturing infrastructure, maintaining the same fill-finish platform. This minimal format change requirement results in swifter, simpler changeovers, which, in turn, delivers time- and cost-saving benefits, while crucially avoiding the higher capital investment associated with additional equipment purchases (Figure 6).
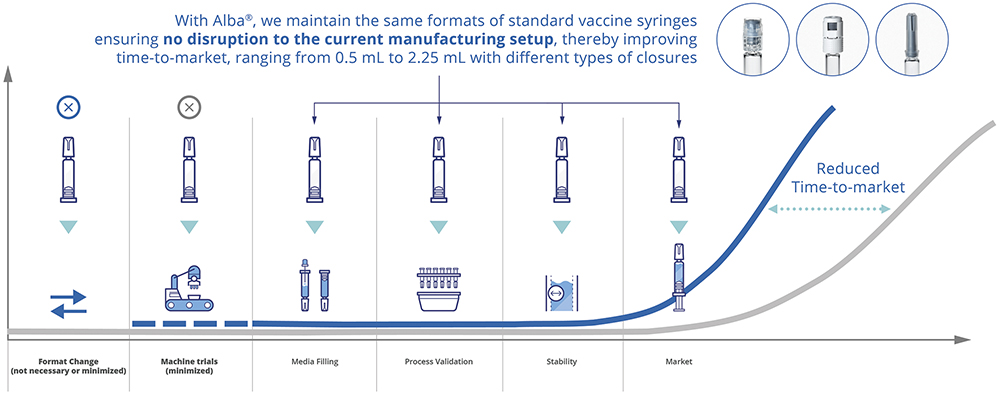
Figure 6: Alba improves time to market by minimising disruption.
“ANOTHER POTENTIAL SOURCE OF DISRUPTION TO CONSIDER FOR mRNA THERAPIES RELATES TO CHANGING CONTAINMENT NEEDS OVER THE COURSE OF A DRUG PRODUCT’S LIFECYCLE.”
Another potential source of disruption to consider for mRNA therapies relates to changing containment needs over the course of a drug product’s lifecycle. For example, vial-based containment solutions might be optimal for bringing a product to market, easing the transition from laboratory to commercial volume production by avoiding the need to validate a different type of container. However, once established, there might be a goal to deliver products in a PFS format ultimately, to support accuracy of dosing and avoid wastage.
CONCLUSION
As mRNA-based treatments and vaccines gain further traction in the market, there is likely to be an escalation in the need both to accommodate transitions in primary packaging and to adapt to changing formulations targeting new medical challenges. At the same time, there will be a constant need to protect the stability of these delicate drug products with high-performance containment solutions. It is a complex dynamic, but one that Stevanato Group’s flexible product offering allows both manufacturing and pharmaceutical partners to resolve without compromise.
Don’t miss the chance to attend the PDA Miniverse Conference on June 25–26, 2025, in Indianapolis, IN, US which will include a tour of Stevanato Group’s new, state-of-the-art US hub. Register for the PDA Miniverse Conference, visit Stevanato Group at booth #218 and join us in Fishers (IN, US) on June 24. Click here for more information: www.stevanatogroup.com/en/news-events/events/pda-miniverse-2025/
REFERENCES
- Swetha K et al, “Recent Advances in the Lipid Nanoparticle-Mediated Delivery of mRNA Vaccines”. Vaccines (Basel), 2023, Vol 11(3), art 658.
- “Gene, Cell, & RNA Therapy Landscape Report: Q4 2024 Quarterly Data Report”. ASGCT & Citeline, Jan 2025.
- Uddin MN, Roni MA, “Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines”. Vaccines (Basel), Vol 9(9), art 1033.
- “Stabilised mRNA vaccine technology aims to end need for frozen storage and improve access”. CEPI, Feb 2023.

