To Issue 175
Citation: Orme L, “Finding Common Ground: Factors for Establishing and Safeguarding Shelf Life in Combination Products”. ONdrugDelivery, Issue 175 (Jul 2025), pp 32–35.
Lauren Orme discusses the regulatory considerations that need to be taken into account when designing combination products. She reviews the essential testing and standards required for both drug and device when establishing their shelf life and storage in a combined product.
For more than half a century, combination products have opened up new and more effective treatment pathways by integrating drugs into complementary delivery platforms. The first combination products, such as antibiotic-loaded bone cements and drug-eluting stents, emerged in the 1970s to lay the foundations for continued advances in materials engineering and drug delivery technology, which prompted significant market growth.1
Indeed, the global annual volume of prefilled syringes alone is estimated currently at around five billion units, with this sector of the market projected to be worth more than $35.7 billion by the end of 2031.2
Where the components of drug and device come together as combination products, whether co-packaged or developed as a single entity, they can bring significant benefits to patients. This is particularly true in the face of rising chronic disease indications, with combination products either simplifying self-administration or easing the treatment burden by enabling drugs to be delivered without the intervention of a medical professional, and often in a home setting.
“A KEY AREA OF CONSIDERATION IS STORAGE AND SHELF LIFE, GIVEN THE FUNDAMENTAL REQUIREMENT FOR A COMBINATION PRODUCT TO FUNCTION SAFELY, EFFECTIVELY AND AS INTENDED WHEN IT REACHES THE POINT OF USE.”
Making these benefits a reality, however, requires manufacturers of combination products to consider and align a range of critical variables. A key area of consideration is storage and shelf life, given the fundamental requirement for a combination product to function safely, effectively and as intended when it reaches the point of use. The shelf life of a combination product can be influenced by various environmental factors including, but not limited to, temperature, humidity, light exposure and pressure. The evaluation of each environmental factor must then be considered during the combination product’s transportation, storage and use.
Therefore, establishing robust shelf-life and stability studies is crucial to the combination product development process and a fundamental requirement for regulatory submissions. Shelf-life requirements are defined by global consensus standards and country-specific regulations. In the case of sterile products, container closure integrity studies will also be required to show that packaging components fulfil their responsibility of maintaining a sterile barrier, and ensuring that the product continues to be protected from contamination, defects or damage. The resulting data from those studies allows accurate labelling information to be established, defining the expiration date.
REGULATORY LANDSCAPE
Globally, findings from well-defined shelf-life studies are vital to provide regulators with evidence to substantiate the labelled storage conditions for the entirety of a product’s stated shelf life. At a practical level, when conducting compliance studies, manufacturers are led by several global consensus standards and widely accepted guidelines from the US FDA, WHO, EU EMA, US Pharmacopoeia (USP) and International Council for Harmonisation (ICH).
For medical devices, widely recognised standards such as ISO 11607, which specifies requirements for real-time ageing of the packaging system, and ASTM d4169, which defines the testing needed to evaluate shipping and transportation,3,4 play critical roles. In addition, ISO 14971 requires full evaluation of the risks present throughout all phases of the lifecycle of a medical device “from the initial conception to final decommissioning and disposal”. Similarly, on the drug side, USP <1207.1> emphasises evaluation of container closure integrity throughout “anticipated storage, shipment, and distribution environment”.
In Europe, the European Medical Device Regulation (MDR) 2017/745 − which replaced the Medical Device Directive (MDD) 93/42/EEC in 2021 − does not explicitly define shelf life, but does include a series of requirements that outline expectations. For example, Annex I, General Safety and Performance Requirements, GSPR 6, states: “The characteristics and performance of a device shall not be adversely affected to such a degree that the health or safety of the patient or the user and, where applicable, of other persons are compromised during the lifetime of the device, as indicated by the manufacturer when the device is subjected to the stresses which can occur during normal conditions of use and has been properly maintained in accordance with the manufacturer’s instructions.”5 In the US, device shelf-life expectations are detailed in FDA Guidance on Shelf Life of Medical Devices.6 These rules define shelf life as the term or period during which the device remains suitable for intended use.
For drug manufacturers, both the US and EU rely on the ICH guidelines, which dictate that formal stability studies should be carried out in accordance with risk management processes, including both long-term and accelerated tests. These guidelines underscore the importance of study controls, including a prescribed stability protocol that contains specific product information, environmental conditions (temperature and humidity) and specified tolerances. These studies provide objective evidence of the period during which a drug product is expected to remain within the approved shelf-life specification, provided that it is stored under the conditions defined on the container label.
Furthermore, the USP provides comprehensive guidelines for drug stability, underscoring the importance of validated testing methods, suitable environmental conditions, packaging and microbiological integrity. It defines stability as: “The extent to which a product retains, within specified limits, and throughout its period of storage and use, i.e., its shelf life, the same properties and characteristics that it possessed at the time of manufacture.”7 Although USP provides its own guidelines, it often aligns with other regulatory documents, such as those of the FDA and ICH, ensuring broad compliance.
Overall, the inherent complexity of combination products requires manufacturers to develop holistic shelf-life studies that meet the regulatory requirements of both drug and device, while also considering the latest consensus standards and guidelines. All of this emphasises the need for manufacturers to develop scientific, risk-based approaches to ensure product reliability and patient safety (Figure 1).
Figure 1: The journey to a robust drug product testing programme starts with the components, through preclinical trials and Phase I of the drug development process.
ESSENTIAL GUIDELINES: CONDUCTING EFFECTIVE STUDIES
When determining shelf life, it is crucial to consider the interaction of all constituent elements – drug, device and packaging – not only in isolation but also in terms of how they will function together as a combination product. Studies should be designed to assess all aspects that support the final combination product requirements and label claim. If this includes multiple conditions (for example, temperature ranges of 2–40°C and humidity ranges of 15–85%), it may be necessary to conduct multiple studies in parallel to fully cover the claimed range. It is quite possible that each element will have differing shelf-life characteristics. As such, once they have been independently established, there is a need to identify the threshold levels that are common to all elements. These lowest common denominator values will ultimately dictate the final shelf-life claim for the combination product as a whole (Figure 2). Therefore, it is critical to ensure alignment between drug and device manufacturers on the data needed to establish the combination product shelf life (e.g. labelling, expiry).
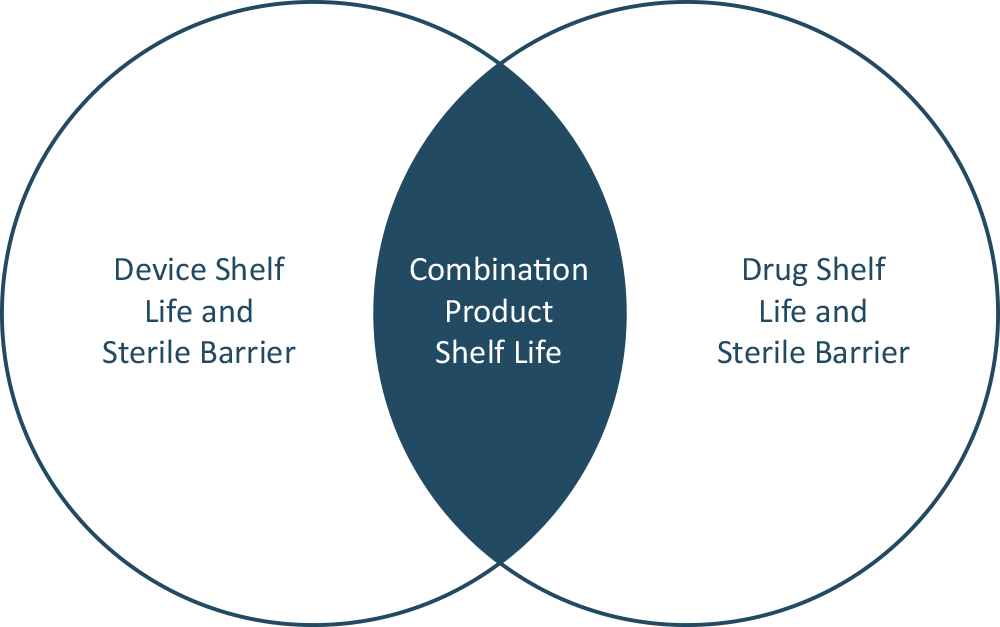
Figure 2: Identifying common shelf-life characteristics between drug and device.
Both functionality and integrity are key areas of focus in the evaluation of factors that could influence shelf life.8,9 When it comes to functionality, products will be assessed as to whether time, use or environmental conditions lead to a compromise in their ability to meet all performance requirements. For example, for drug delivery devices, essential drug delivery outputs (EDDOs) and primary functions should also be considered as part of shelf life. There must also be an assessment of their effects on the sterile barrier, drug stability and container closure integrity (CCI) as per USP <1207> “Package Integrity Evaluation − Sterile Products”.10,11
When generating data to support shelf life, both accelerated and real-time studies can be beneficial to support the overall data package. While real-time data are expected for drug product submissions, most regulatory bodies will accept accelerated data to support medical devices for the initial shelf-life claim, with the expectation that real-time studies are being conducted to confirm the claim.
Significant study controls must be in place for this data to be deemed sufficiently valid. For example, to ensure that results reference equivalent products and batches, studies should be conducted on the “to-be-marketed” version of the product. As such, all processing activities including manufacturing, materials and packaging should be identical to those of the final combination product. Where any differences exist, strong scientific rationales should be generated to provide justification of study validity.
Consideration must also be given to the fact that studies are conducted using qualified, calibrated and validated chambers capable of maintaining the tolerances specified in industry consensus standards. Examples of such tolerances include ±5°C for freezer conditions of -20°C; ±3°C for refrigeration conditions of 5°C; ±2°C for ambient conditions above 8°C; and ±5% for humidity testing at any temperature.
“FOR SHELF-LIFE STUDIES TO BE TRULY ROBUST, IT IS CRITICAL TO CONSIDER NOT JUST TESTING THE FINISHED PRODUCT BASED ON FINAL STORAGE CONDITIONS BUT ALSO TO EVALUATE THE POTENTIAL AREAS THAT COULD NEGATIVELY IMPACT ITS PERFORMANCE TO FUNCTION.”
Importantly, for shelf-life studies to be truly robust, it is critical to consider not just testing the finished product based on final storage conditions but also to evaluate the potential areas that could negatively impact its performance to function as intended throughout its entire lifecycle. Supply chains will all differ in terms of the various stages involved and storage conditions must be considered at every stage, from manufacturing to sterilisation, co-packaging and distribution. Therefore, it is critical to review the supply chain in detail and ensure that studies are conducted to represent the process appropriately.
Since multiple stakeholders are likely to be involved, including distributors and importers, quality agreements should be used to outline roles, responsibilities and obligations to ensure compliance with the previously defined storage conditions based on shelf-life requirements. It is only through such an integrated approach that manufacturers can be sure that shelf-life claims will be upheld all the way throughout the supply chain to the patient.
With multiple actors and variable factors to consider, the complexity of establishing and supporting combination product shelf-life claims becomes increasingly apparent. Far from being regarded as a linear list of tasks to be completed, it demands a highly integrated approach, where the characteristics of interlinked elements are considered, and all parties are collectively aligned on critical parameters dictated by high-integrity data.
West’s direct experience of working with pharmaceutical partners on shelf-life studies has underlined how this process can be enhanced and accelerated by adopting a holistic, data-driven approach. The company’s comprehensive, consultative support in this area covers everything from strategic planning to stability studies, and is underpinned by extensive knowledge of the latest regulatory standards to ensure that labelling claims are robust and fully substantiated. These are services that form part of West’s wider approach to proprietary and non-proprietary combination products, which is backed by its deep understanding of primary packaging components and systems, and a broad, integrated offering that includes in-house analytical testing as well as services relating to clinical development and regulatory compliance.
REFERENCES
- Tian J et al, “Regulatory perspectives of combination products”. Bioact Mater, 2021, Vol 10, pp 492–503.
- Jansen-Otten C, Zeiss B, “All about Pre-filled Syringe Systems”. Presentation, PDA Parenteral Packaging Conference, Mar 2025, Málaga, Spain.
- “Packaging for terminally sterilized medical devices Part 1: Requirements for materials, sterile barrier systems and packaging systems”. ISO 11607-1:2019.
- “Standard Practice for Performance Testing of Shipping Containers and Systems”. ASTM D4169-22, Feb 2024.
- “ANNEX I – General safety and performance requirements”. EU MDR, 2019.
- “Shelf Life of Medical Devices”. Guidance Document, US FDA, Apr 1991.
- “Stability Considerations in Dispensing Practice”. USP <1191>.
- “Draft Guidance for Industry: Essential Drug Delivery Outputs for Devices Intended to Deliver Drugs and Biological Products”. US FDA, Jun 2024.
- “Needle-based injection systems for medical use – Requirements and test methods – Part 1: Needle-based injection systems”. ISO 11608-1:2022.
- “Package Integrity Evaluation – Sterile Products“. USP <1207>, 2016.
- DeGrazio F, “USP Chapter 1207: Package Integrity Evaluation – Sterile Products”. Company Blog, West Pharmaceutical Services, Jul 2016.

