To Issue 152
Citation: Oakley T, Jameson H, “Child is Not a Small Adult: Challenges and Solutions in Developing Drug Delivery Devices for Children”. ONdrugDelivery, Issue 152 (Oct 2023), pp 80–82.
Tom Oakley and Heather Jameson consider the main differences in developing therapies and drug delivery devices for children and babies when compared with adults.
Paediatricians have been known to say, “a child is not a small adult!” Why do they say this, and what does it mean for developing therapies and drug delivery devices?
This article itemises the main differences between adults and children and the implications, including:
- Anatomical development
- Metabolism and pharmacokinetics
- Patient ability and usability
- Patient preference and adherence.
“The differences in anatomy between children and adults, as well as between anatomical ages, have many implications for drug delivery devices.”
ANATOMICAL DEVELOPMENT
The anatomical development of the child is critical when considering therapies and devices. Apart from the obvious changes in height and weight over time, there are more subtle changes that occur as the child grows. For example, the sitting height of a newborn infant represents about 70% of total body length but, by the age of three years, this typically reduces to 57% and, as an adult, typically 50%. In newborn infants, muscle mass constitutes approximately 25% of body weight compared with 43% in adults.1
Growth and development are not constant processes for the individual, and they vary greatly between individuals of the same age. For newborn children, gestational age (age since conception) can be more meaningful than age since birth.
Nasal anatomy is an example where key differences exist between children and adults. Infants under two years old also have more diffuse lymphoid follicles throughout the nasal cavity in addition to the nasal-associated lymphoid tissue areas found in the adult nasal cavity.2 Infant nasal anatomy has a greater degree of similarity to rodents than that of adult humans, meaning that the results of studies with rodent models could be more applicable for human infants. This could theoretically help to accelerate development of infant nasal vaccines; however, in practice safety and efficacy are generally established in adults before any infant studies commence for reasons we will cover later.
The differences in anatomy between children and adults, as well as between anatomical ages, have many implications for drug delivery devices. For example, emergency autoinjectors that inject into the muscle need to account for the difference in fat and muscle distribution in children, and inhalers need to be appropriate for the smaller lung capacity and inspiratory flow rates in children. Some injection sites that are suitable for adults might be unsuitable for young children.
METABOLISM AND PHARMACOKINETICS
Children have a reduced ability to maintain homeostasis compared with adults due to their greater metabolic and nutritional requirements (relative to their body mass). Most of the first year of life is characterised by immaturity of kidney and liver function, so younger children are more susceptible to dehydration, fever and other conditions. Therefore, drug delivery devices may need to allow for more regular delivery of smaller doses than for adults, or even quasi-constant drug administration, possibly under the supervision of a clinician. Dose increments may need to be smaller for children than adults.
“When most new medicines are licensed or receive their marketing authorisation, they are only licensed for use in adults.”
The reticuloendothelial system, composed of macrophages found in the lymph nodes, spleen and other lymphatic tissues, is comparatively more active in childhood. Lymphatic tissues, such as tonsils and adenoids, swell rapidly in response to minor infections. Antibody production is different in infants compared with older children or adults. Thus, drugs that influence the immune system, such as immunosuppressant monoclonal antibodies, may need to be given in different doses, to different parts of the anatomy (for example, multiple injection sites), with different pharmacokinetic modifiers (such as sustained-release formulation or even implants).
When most new medicines are licensed or receive their marketing authorisation, they are only licensed for use in adults. This is because, on the whole, the manufacturer only investigates their safety and efficacy in the adult population. This could be due to a lack of commercial incentive for conducting studies in children or an inability to recruit enough children for clinical trials. In 2004, the UK government recognised the need for high-quality research in children and young people and resolved to establish a national research network to study medicines for children.3
PATIENT ABILITY AND USABILITY
There is a widespread trend across healthcare to move treatments from the hospital to the general practitioner, and from the general practitioner to self-administration. Reasons for this are well documented and tend to focus on patient convenience and reduction of healthcare costs.
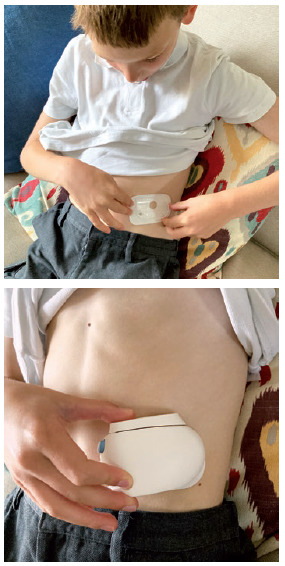
Figure 1: Child positioning a wearable injector on his stomach, ready for use.
However, there are additional considerations when treating children compared with most adults.
The ability of the child to self-administer drugs is likely to be limited, especially for younger children. In the first few years of life, “the user” is going to be an adult, for example, a parent, other carer or clinician. The separation of “user” from the “patient” means that device design might need to be modified. Imagine an on-body delivery system where the user interface is designed for the patient to operate but, in this case, somebody else must operate it. A display or icon designed for the patient to see when they look down at their stomach will be upside down when viewed by a user who is facing the patient (Figure 1).
Training and instructions for use can be more complicated for treating children because multiple people (child, parent, clinician, etc) may need to be involved, each having different roles and varying levels of life experience and learning needs. A study of the readability of manufacturers’ patient information leaflets for commonly used medicines for children showed that nine out of 10 could not be read at a reading age of 13. Indeed, many adults would not be able to use those instructions because 9% of adults have a reading age of 13 or lower.3
Young children may find it more difficult to alert adults to adverse events such as pain, nausea or swelling, and so extra care must be taken to ensure that the drug delivery device and drug are not causing excessive side effects.
Co-ordination of movement can be harder to achieve for children than adults. Several studies have shown that adults struggle to co-ordinate the activation of a pressurised metered dose inhaler with their inhalation manoeuvre. The smaller lung capacity and inspiratory flow rate of children mean that the optimal activation window is even smaller. Therefore, many children need to use a spacer with a pressurised metered dose inhaler.4 Even then, around 20% of children under two years old cannot generate the inspiratory pressure to open a unidirectional valve of a valved holding chamber (Figure 2).5

Figure 2: Child using a spacer to aid drug delivery to the lungs.
“The ability of the child to self-administer drugs is likely to be limited. In the first few years of life, ‘the user’ is going to be an adult, for example, a parent.”
PATIENT PREFERENCE AND ADHERENCE
Medical devices for adults are tending to look increasingly like consumer products due to patient preference (such as fitting with their lifestyle image) and user interface expectations.
Children are likely to have different preferences and values from adults. Some pharmaceutical and medical device companies have made progress in adapting devices to appeal to children, for example, by having superhero or other graphic themes on the device.
Some companies have considered adding fun or engaging features to drug delivery devices that could motivate children to adhere to the instructions for use, for example, adapting an inhaler to play a sound when inhaling correctly. Others have added “gamification” to devices so that children feel more engaged, motivated and positive about interacting with their therapy regime.
There is evidence of adverse consequences because of low compliance in children in prophylactic treatments, especially in antibiotics, asthma, epilepsy and lymphoblastic leukaemia. Adolescence poses a special challenge concerning treatment compliance: the evidence shows low rates of adherence in cystic fibrosis, diabetes and asthma.3
SUMMARY
We have seen how the differences in the anatomy, metabolism, ability and preferences of children demand customisation of drug delivery devices to meet their needs. Deep understanding of the patients and users (who are often different people) is necessary when delivering drugs to children and babies. Research and formative studies need to be conducted early and throughout device development, and the treatment regime and device characteristics may need to be different depending on patient age.
REFERENCES
- “Disease-affecting differences between children and adults”. Britannica.com, accessed Aug 2023.
- Cai L, Xu H, Cui Z, “Factors Limiting the Translatability of Rodent Model–Based Intranasal Vaccine Research to Humans”. AAPS PharmSciTech, 2022, Vol 23, p 191.
- “Medicines for Children and Young People”. Department for Education and Skills, Oct 4, 2004.
- O’Callaghan C, Barry PW, “How to choose delivery devices for asthma”. Arch Dis Child, 2000, Vol 82, pp 185–191.
- Vincken W, Levy ML, Scullion J et al, “Spacer devices for inhaled therapy: why use them, and how?” ERJ Open Research, 2018, Vol 4, pp 00065–2018.

