To Issue 169
Citation: Abedian R, Stockton M, Zeiss B, “A Comprehensive Approach to Addressing Unmet Needs in Intravitreal Injections”. ONdrugDelivery, Issue 169 (Mar 2025), pp 6–11.
Reza Abedian, Marie Stockton and Bernd Zeiss consider the complexities and clinical unmet needs of intravitreal injections and discuss how the Gerresheimer’s syringe technology can help overcome these challenges.
Treatment of retinal disease has been revolutionised thanks to the development of protein-based liquid drugs delivered directly into the vitreous body via intravitreal injection (IVI). Such injections of precise drug concentrations directly to the posterior eye segment can minimise systemic side effects.1 However, despite the clinical efficacy of these therapies, there are challenges associated with drug delivery via IVI, including precision and repeatability of the injection dose volume, procedural inefficiency and patient safety.2
To address these challenges Gerresheimer has conducted extensive research and testing to provide specific syringe technologies optimised for IVI. The company is also committed to ongoing innovation in this field to provide even greater support to clinicians and patients.
THE ROLE OF IVIs IN TREATING RETINAL DISEASES
IVIs have become the standard practice for administering anti-vascular endothelial growth factor (anti-VEGF) drugs, corticosteroids and antibiotics to treat retinal conditions. This approach is critical for managing a number of serious conditions. Wet age-related macular degeneration (AMD), one of the leading causes of vision loss in older adults, is treated through anti-VEGF therapies, which inhibit the action of VEGF, reducing neovascularisation and fluid leakage, thereby stabilising or improving vision in many patients. Similarly, diabetic macular oedema, diabetic retinopathy and retinal vein occlusion are commonly treated through IVIs to reduce macular swelling and restore or maintain visual acuity. With a growing prevalence of patients with diabetes and an ageing population in most geographical regions, the number of IVIs is increasing year on year, and the demand for efficient and effective IVIs is growing accordingly.
“THE SYRINGE USED FOR THE IVI IS A CRITICAL FACTOR, BUT THE PHYSICIAN’S PRACTICAL EXPERIENCE ALSO HAS A SIGNIFICANT INFLUENCE ON ACCURATE DOSING.”
CHALLENGES IN INTRAVITREAL INJECTIONS
Dose Volume Accuracy and Reproducibility
As IVIs are usually administered in volumes of between 20 to 100 μL, accurate and reproducible microlitre dosing is crucial. The syringe used for the IVI is a critical factor, but the physician’s practical experience also has a significant influence on accurate dosing,3 as moving the plunger the correct amount while removing the air bubble and priming the syringe can impact significantly on injected volume. This is particularly challenging with very small volumes, as the plunger only moves a tiny distance. Moreover, the user is only guided by a visual marking (usually a dark ring) on the outer syringe barrel to set the injection dose volume. In a study assessing 800 injections, up to 22% of injections with a prefilled syringe (PFS) deviated by 20% or more from the intended volume of 0.05 mL.4
Patient Safety and Treatment Outcome
As discussed, precise administration is a challenge when delivering small volumes. If too much of the drug is injected it could result in complications such as intraocular inflammation, haemorrhage and elevated intraocular pressure (IOP), also referred to as postoperative IOP spikes.5 These risks are compounded when repeated injections are required for chronic conditions, such as AMD. Underdosing as a result of variability in technique, as well as large dead space in some syringes, could also be problematic, as it may negatively impact the therapeutic effect while also leading to wastage of an expensive drug formulation.
Another concern is particles entering the vitreous body during injection. This is particularly relevant when treating conditions that require repeated injections, which could lead to a build-up of particles, often referred to as floaters, within the eye over time and potentially affect vision. Particle count is therefore subject to strict regulations in accordance with USP <789> and Ph Eur 2.9.19. One of the main causes of particles is migration into the eye is silicone oil used to lubricate the inside of the syringe. Other sources include the drug formulation, the administration process and the plunger stopper.
The frequently used process of transferring the drug from a vial to a regular spray-siliconised disposable syringe is an additional source of increased particle count. The use of a PFS contributes significantly to lower residual volume and lower particle loads while significantly reducing the risk of contamination and, consequently, endophthalmitis.6,7
Procedure Efficiency and Clinician Burden
The current practice of intravitreal injections can be time consuming, contributing to higher costs and resource burdens in clinical settings. If the therapeutic drug is stored in a vial, it first has to be transferred to a syringe and primed for the patient-intended dose prior to administration. This opens up the possibility of manual error and also impacts efficiency, which becomes ever more important with the increasing prevalence of retinal diseases requiring treatment delivery via IVIs.
SOLUTIONS TO ADDRESS CHALLENGES OF IVI
Gerresheimer is one of the leading suppliers of primary packaging to the ophthalmic market. The company is committed to investing in ongoing research and development of solutions to fulfil unmet needs in this field.
The first stage of eliminating workflow steps is to opt for a PFS (Figure 1). In this case, the drug is prefilled into a glass or cyclo-olefin-polymer (COP) syringe in the correct volume and dose concentration with no need to be transferred into a syringe from a vial. Not only does the use of a PFS streamline workflow but it also reduces potential errors and the possibility of contamination. Most importantly, recent studies show that PFS use was associated with a significant reduction in the rate of endophthalmitis following IVIs.7

Figure 1: 0.5 mL glass syringes for ophthalmology with Luer lock adapter, available BOS or silicone-oil-free, ready-to-fill (RTF®) format, various elastomer components and dose mark options.
BOS Syringes
Unlike standard spray-siliconisation methods, which are suitable for many drugs and applications, only a special low-particle siliconisation, such as baked-on siliconisation (BOS), provides a very thin, more uniform and stable silicone layer as the silicone oil is bonded to the surface via hydrogen bonds and partly covalent bindings. This significantly reduces the risk of silicone oil droplets migrating into the drug over its shelf life and during injection and potentially causing aggregation, drug interaction and particle migration into the vitreous body. BOS has proven to support USP <789> requirements of the final ophthalmic drug product.
“GERRESHEIMER’S INTRODUCTION OF SILICONE-OIL-FREE SYRINGES MARKED A PIVOTAL INNOVATION IN SYRINGE TECHNOLOGY.”
Silicone-Oil-Free Syringes
Gerresheimer’s introduction of silicone-oil-free syringes marked a pivotal innovation in syringe technology. These syringes do not use silicone as a lubricant, which eliminates silicone-derived particle formation. Silicone oil can induce the formation of protein aggregates prior to IVI. The release of silicone from the syringe can exacerbate this process, leading to increased levels of protein particles in the formulation.8 Therefore, elimination of silicone supports greater compatibility with sensitive protein-based drugs by reducing the likelihood of aggregation.9
Proven Syringe Performance
In a study evaluating BOS and silicone-oil-free syringes, particle levels were found to be significantly below USP <789> requirements, confirming their suitability for ophthalmic applications (Figure 2). Testing involved the use of water for injection in both glass and COP barrels under real-time and accelerated storage conditions. Results indicated consistently low particle counts across all scenarios. Comparative analyses between BOS and silicone-oil-free syringes revealed both options to be highly effective and compliant with USP <789> requirements.
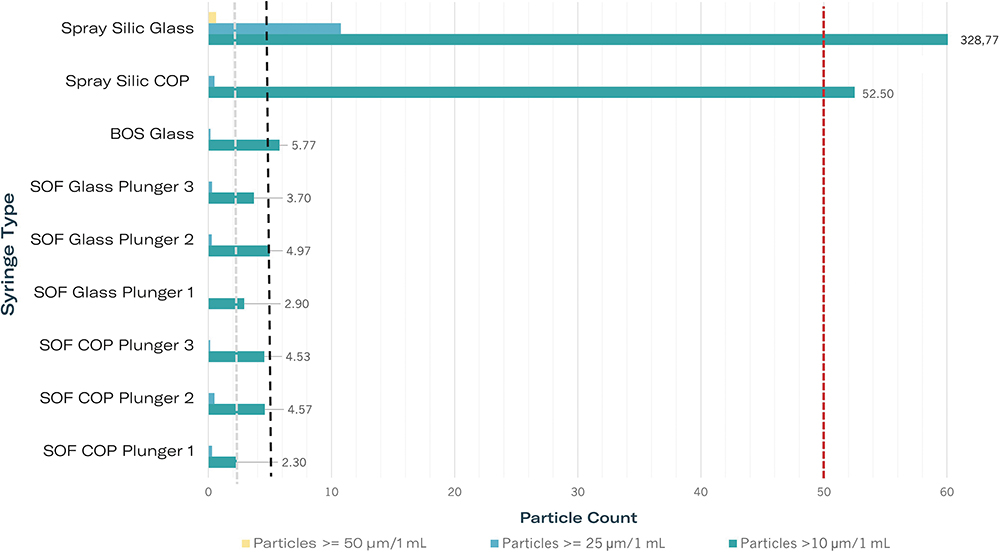
Figure 2: Particle measurements of silicone-oil-free 1-mL-long syringes compared with siliconised (Silic) systems in accordance with USP <789>. Dashed lines indicate limits for three particle classes in accordance with USP <789>. All syringes filled with water for injection. Legend: COP/glass – syringe body material; Spray Silic – spray siliconised, BOS – baked-on siliconised, each with modern coated plunger stoppers; SOF with plungers 1–3 – silicone-oil-free syringes with various plunger stoppers.
Dose Accuracy
For delivery of a precise microlitre dose, PFSs of 0.5 or 1 mL are required due to their small internal diameter. Tolerances for the internal dimensions of glass syringes can be set to ± 0.1 mm or even ± 0.05 mm to meet the most critical requirements. The moulding process used in manufacturing of the COP syringes means that these have even tighter tolerances contributing to higher dose-volume accuracies.
Dose marking is a key visual aid for retinal specialists when administering such small dose volumes. The visual dose mark helps to assess the stop position of the plunger for precise administration of the patient-intended dose. Gerresheimer employs a cutting-edge camera-based inspection system within its glass syringe production plants, which ensures accurate dose marking on its syringes with tolerances as low as ± 0.25 mm.
Break-Loose and Gliding Forces
Break-loose and gliding force (BLGF) tests further validate the functionality of these syringes for IVIs. After three months of accelerated ageing (equivalent to three years of real-time storage), all silicone-free glass syringes demonstrated BLGF values below 20 N, indicating excellent usability. The absence of ageing effects on gliding forces highlights the stability and reliability of the syringe systems (Figure 3).
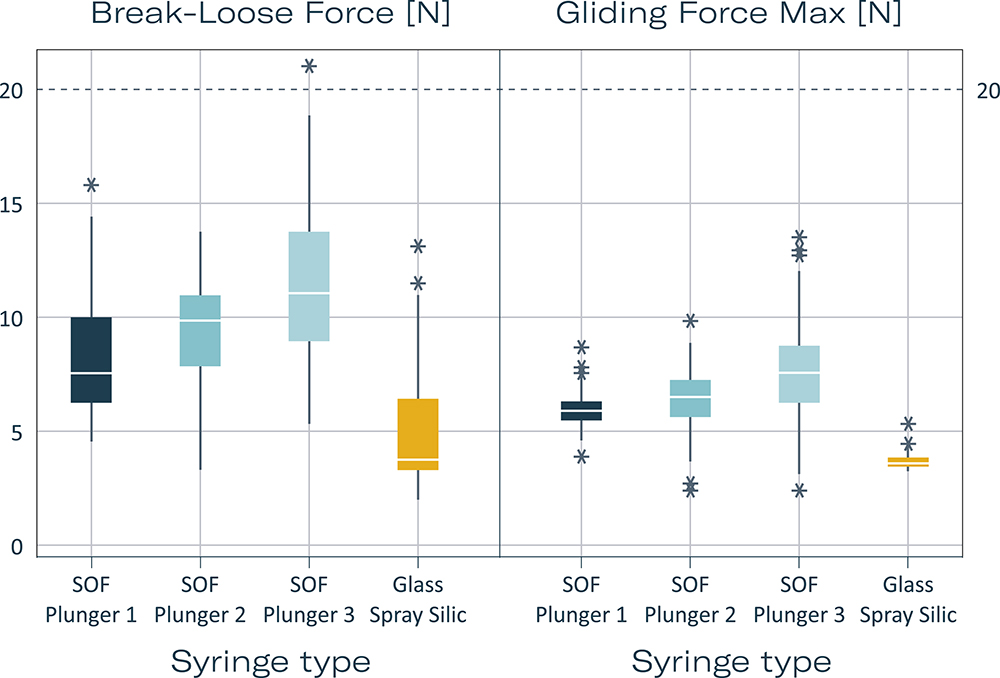
Figure 3: Break-loose and gliding forces of silicone-oil-free syringes compared with spray-siliconised syringes. Extrusion force 270 mm/min. Each syringe 1 mL long with 27 G needle syringe with standard ID, filled with water for injection. Measurement times each with N = 160: summed [T0 (3 days after filling), T1 3 months, T1 acc (3 months accelerated ageing in accordance with ICH), T2 acc (6 months accelerated ageing in accordance with ICH), T2 (6 months)]. Legend: SOF with plungers 1–3: Silicone-oil-free syringes with three special plunger stoppers from different manufacturers; Glass Spray Silic – 0.5 mg silicone oil, coated plunger stopper.
A central focus on improving the experience of both patients and clinicians during IVI procedures while driving innovation is a top priority for Gerresheimer. By reducing or eliminating the presence of silicone particles and ensuring smooth injection by PFSs, the company’s solutions contribute to higher safety levels for patients. For clinicians, the use of PFSs simplifies the preparation process, reducing procedural time, and the risk of dosing errors and contamination. This not only enhances workflow efficiency but also allows healthcare professionals (HCPs) to focus more on patient care.
CAN IVI PRACTICE BE FURTHER OPTIMISED?
Gerresheimer’s syringes are shown to minimise the challenges of conventional IVIs to a great extent. However, the judgement, dexterity and experience of the retina specialist continues to have an influence on the accuracy of microlitre dosing, reproducibility and patient comfort.10 These variables are particularly critical with very small volumes – for example, the treatment of paediatric patients with retinopathy of prematurity is likely to involve volumes as small as 20 μL. In this case, patient comfort and safety is also of greater concern. Another consideration is the therapeutic window of the drug. If this is very narrow, over- or underdosing could have a greater influence on therapy effectiveness. Gerresheimer therefore instigated an innovation project to investigate the potential of an injection-aiding device to further optimise IVIs for patients and HCPs.
User-Centric Research
User centricity is a cornerstone of Gerresheimer’s development process. Whenever a potential unmet need is identified, Gerresheimer’s first step is to initiate a user preference study. In this instance, a combination of an online survey and in-person interviews were conducted with 25 retina experts to confirm challenges and identify further unmet needs.11 This phase of research was followed by in-person interviews with international key opinion leaders (n = 5). The results confirmed that ease of handling, precise needle positioning, accuracy and consistency of dose delivery are viewed as critical factors by HCPs.
Initial Concept Testing
As a result of the initial research, several potential device concepts were generated that focused on the unmet needs of users, such as handling characteristics and accuracy of intended injection volume. Verification testing and user experience validation of potential device concepts were carried out. Both qualitative evaluation and statistical analysis were used to determine significant differences between the results of injection of a fixed intended dose with a chosen device concept compared with standard of care with a conventional PFS. Injection volumes, measured while using an injection-aiding device, demonstrated less dispersion around the intended dose volume compared with those performed with a PFS only (Figure 4).
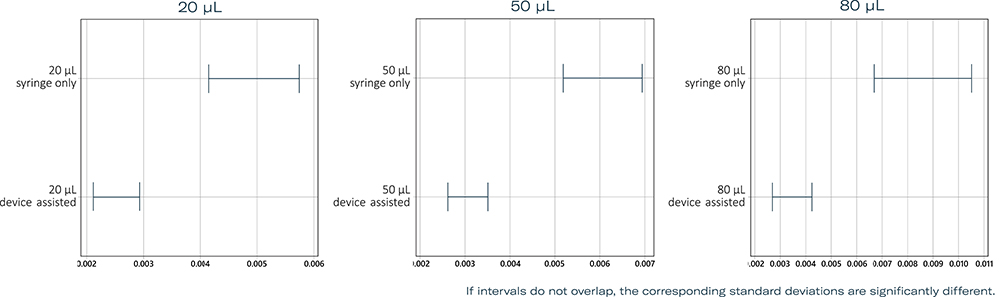
Figure 4: Results of tests for equal variances at target volumes of 20, 50 and 80 μL with standard practice using a PFS compared with an injection-aiding device. Multiple comparison intervals for the standard deviation, α = 0.05.
In-Depth Prototype Testing
An evolved prototype of the preferred device solution was then produced and subsequently underwent rigorous testing to assess injection accuracy and repeatability across target doses of 20, 50 and 80 μL. Five users performed injections of each of the target dose volumes using both the injection device prototype with PFS and the current standard of care in IVI with a PFS using a visual dose marker (n = 10 injections per user and target dose volume) (Figure 5).
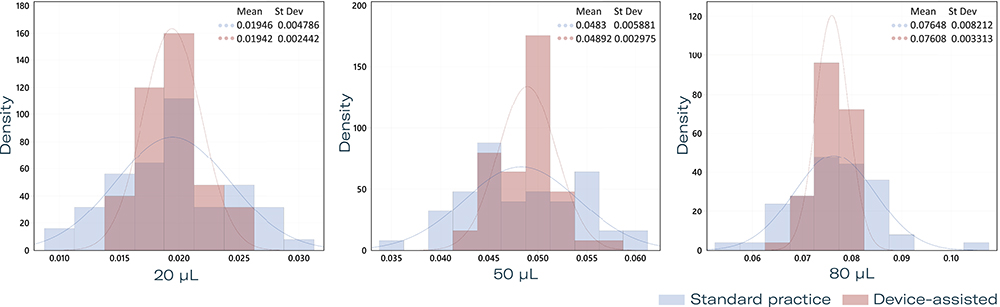
Figure 5: Histogram of injection volumes of 20, 50 and 80 μL with standard practice using a PFS compared with an injection-aiding device.
Results showed reduced variability in delivered volume with the injection device compared with manual injection. Statistical analyses confirmed the superior consistency of device-assisted injections. The potential benefits of this device are manifold; by enhancing key aspects of the injection process, an injection-aiding device could increase patient safety by minimising dosage errors. It also has the potential to reduce procedural time, benefiting both patients and clinicians.
“GERRESHEIMER REMAINS COMMITTED TO WORKING CLOSELY WITH EXPERTS IN THE FIELD TO ENSURE THAT THE FINAL PRODUCT MEETS THE HIGHEST STANDARDS OF SAFETY AND EFFICACY.”
Gerresheimer remains committed to working closely with experts in the field to ensure that the final product meets the highest standards of safety and efficacy.12 Future development steps include further testing to refine the device design and validate its performance under real-world conditions. The research project is ongoing and the next results have been accepted for presentation at the Association for Research in Vision and Ophthalmology annual meeting (ARVO) on May 4–8, 2025 in Salt Lake City (UT, US).
CONCLUSION
IVIs are indispensable in ophthalmology, yet challenges remain in ensuring precise, safe and efficient administration, particularly in demanding retinal therapies. Gerresheimer’s ongoing commitment to patient and user-centric innovation ensures that the company continuously strives to identify and fulfil unmet needs. In this way, the company remains at the forefront of solutions for intravitreal drug delivery, aiding healthcare providers and, ultimately, contributing to optimal outcomes for patients.
REFERENCES
- Angermann R et al, “Changes in systemic levels of vascular endothelial growth factor after intravitreal injection of aflibercept or brolucizumab for neovascular age-related macular degeneration”. Retina, 2022, Vol 42(3), pp 503–510.
- Agra LM et al, “Accuracy, Precision, and Residual Volume of Commonly Used Syringes for Intravitreal Injections and the Impact on Intraocular Pressure”. Ophthalmol Retina, 2023, Vol 7(10), pp 892–900.
- Meyer CH et al, “Routes for Drug Delivery to the Eye and Retina: Intravitreal Injections”. Dev Ophthalmol, 2016, Vol 55, pp 63–70.
- Raju JR and Weinberg DV, “Accuracy and precision of intraocular injection volume”. Am J Ophthalmol, 2002, Vol 133(4), pp 564–566.
- Dounce S et al, “Particulate Matter from Syringes Used for Intravitreal Injections”. Retina, 2020, Vol 41(4), pp 827–833.
- Levin AM et al, “Intraocular Pressure Elevation Following Intravitreal Anti-VEGF Injections: Short- and Long-term Considerations”. J Glaucoma, 2021, Vol 30 (12), pp 1019–1026.
- Louis AM et al, “Impact of Prefilled Syringes and Masking on Postintravitreal Injection Endophthalmitis”. J Vitreoretin Dis, 2023, Vol 7(5), pp 382–388.
- Melo GB, “Potential Implications of Silicone Oil From Syringes.” Retinal Physician, 2022, Vol 19, pp 40–42.
- Schargus M et al, “Contamination of anti-VEGF drugs for intravitreal injection: How do repackaging and newly developed syringes affect the amount of silicone oil droplets and protein aggregates?” Retina, 2018, Vol 38(10), pp 2088–2095.
- Shetty G, Zeiss B, “Microlitre Dosing with Prefillable Syringes – When Does a Device Make Sense?”, ONdrugDelivery, Issue 97 (May 2019), pp 28–31.
- Abedian R et al, “Facilitating microliter dosing for intravitreal application: A User-Preference Study”. Gerresheimer, Oct 2023.
- Abedian R et al, “A User-preference Study on an Ophthalmic Injection Device to Facilitate Microliter Dosing for Intravitreal Injections”, Proc PDA Universe of PFS and Injection Devices Conference, Phoenix, AZ, US, Oct 22–23, 2024.

