To Issue 169
Citation: Prabhathachandran A, Peretz M, Potnis M, “A New Delivery Platform for Suprachoroidal Administration Using West’s Advanceable Microneedle Device”. ONdrugDelivery, Issue 169 (Mar 2025), pp 14–18.
Anu Prabhathachandran, Menachem Peretz and Manali Potnis discuss the growing need for more effective delivery of therapeutics to treat ophthalmic conditions to meet the needs of the ageing population, including how delivery into the suprachoroidal space may present an appealing alternative to intravitreal injection. The authors go on to introduce West Pharmaceutical Services’ Suprachoroidal Advanceable Microneedle Device, which offers several advantages for overcoming the challenges with suprachoroidal space.
As the population ages, the prevalence of eye diseases, such as age-related macular degeneration (AMD), geographic atrophy and diabetic retinopathy, is rising significantly. By 2040, AMD will affect 288 million patients worldwide.1 By 2030, approximately 14 million patients globally will be treated with intravitreal injections (IVIs) for retinal diseases.2 With a patient receiving around 4–8 injections per year, this translates to approximately 112 million injections annually.2 Without proper adherence to these maintenance injections, patients face the risk of irreversible vision loss, and thereby their independence.
Since the approval of intravitreal ranibizumab (Lucentis®, Genentech) in 2014, intravitreal delivery has become a predominant method for ocular drug administration, with around 10 million IVIs administered annually in the US.3 Despite its widespread use, intravitreal delivery has risks of intraocular inflammation when used to deliver newer therapeutics, such as gene therapies. IVIs risk systemic inflammation due to the leakage of drugs into systemic circulation and have limited efficacy in delivering therapies to the inner retina across restrictive biological barriers.4 The alternative – sub-retinal injection – is effective but requires complicated, invasive surgical procedures that are not feasible for widespread use in a busy retina clinic.
“SUPRACHOROIDAL DELIVERY OFFERS A PROMISING ALTERNATIVE ROUTE OF ADMINISTRATION BY ENABLING TARGETED DRUG DELIVERY THROUGH A MINIMALLY INVASIVE IN-OFFICE PROCEDURE.”
Suprachoroidal delivery offers a promising alternative route of administration by enabling targeted drug delivery through a minimally invasive in-office procedure. This method involves delivering drugs into the potential space between the sclera and the choroid, enabling medication to travel posteriorly and circumferentially to the retina. Currently, only one small molecule drug is approved for suprachoroidal delivery, which offers comprehensive coverage of the choroid and the retina compared with conventional IVIs.5
Currently, several gene therapies and small molecule-based therapies targeting the suprachoroidal space are being evaluated in various clinical trials.5 Small molecules can access the diseased area of the retinal pigment epithelium (RPE) and choroid, whereas large molecules cannot pass through Bruch’s membrane and are therefore limited to the RPE side.5 The apposition of the drugs on the choroidal layer may offer significant benefits in controlling inflammation in geographic atrophy, with specific advantages for extended delivery systems that can offer more durability to the therapy. The potential for improving therapeutic outcomes through better tissue access, physician training and patient education is significant in this route of administration.
“DESPITE THE POTENTIAL ADVANTAGES, ACCESSING THE SUPRACHOROIDAL SPACE IS VERY CHALLENGING WITH CURRENT CONVENTIONAL PRACTICES DUE TO THE ANATOMICAL VARIABILITY AND THE COLLAPSED STATE OF THE SUPRACHOROIDAL SPACE.”
Despite the potential advantages, accessing the suprachoroidal space is very challenging with current conventional practices due to the anatomical variability and the collapsed state of the suprachoroidal space. Notable challenges in accessing the suprachoroidal space include maintaining consistent pressure on the sclera to dimple the tissue during injections and the longer injection duration compared with intravitreal procedures – 5–10 seconds per eye for the suprachoroidal space versus approximately 1 second for an IVI.5 The need for slow injection is to minimise the pain experienced by the patients during the injection. For suprachoroidal injection, dimpling is necessary to clear the conjunctiva and expand the suprachoroidal space.
These challenges result in injections failing in approximately 30% of patients, and a second injection with a longer microneedle is required to access the space.6 Applying slightly greater pressure with the needle on the ocular surface prior to switching to a different quadrant or to a longer needle also increases patient discomfort during the injection.
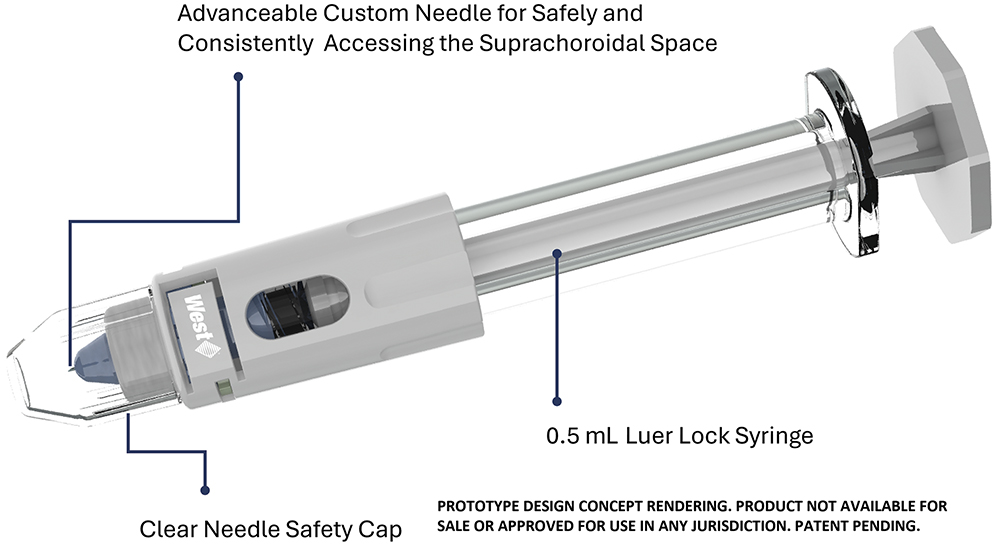
Figure 1: Render of West’s Suprachoroidal Advanceable Microneedle Device.
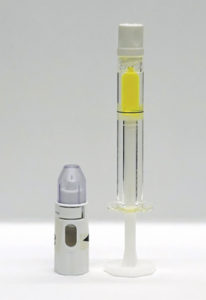
Figure 2: Suprachoroidal Advanceable Microneedle Device prototype and 0.5 mL prefilled syringe with surrogate drug product. Prototype design not available for sale or approved for use in any jurisdiction.
INTRODUCING THE SUPRACHOROIDAL ADVANCEABLE MICRONEEDLE DEVICE
To accommodate the anatomical variability of individual patients and find a solution for the challenges discussed above, West Pharmaceutical Services has introduced the Suprachoroidal Advanceable Microneedle Device (Figures 1 & 2). This device features firm dimpling followed by incremental needle advancement, allowing for more precise delivery of therapeutics into the suprachoroidal space (Figure 3). The goal of the device and the advancement feature is to increase the precision and accuracy of suprachoroidal delivery by minimising the need for any unnecessary manoeuvring or excessive dimpling. This compensates for the depth needed in accessing the tissue, thereby avoiding the need for the second injection that arises in approximately 30% of cases using conventional practices.6 The anticipated benefits of the new platform are summarised here:
- Single stick
- Faster injections
- Potential for reduced pain during injection
- Perpendicular access, which is easier to train and implement
- Potential to use the advancement feature to access the suprachoroidal space without rocking or twisting (manoeuvres that may increase the risk of choroidal haemorrhage)
- Potential to use a prefilled syringe with accurate dose marking
- Standard plunger rod platform similar to the devices used for IVIs, which may have a distinct advantage for both surgeon preference and drug manufacturer adoption.
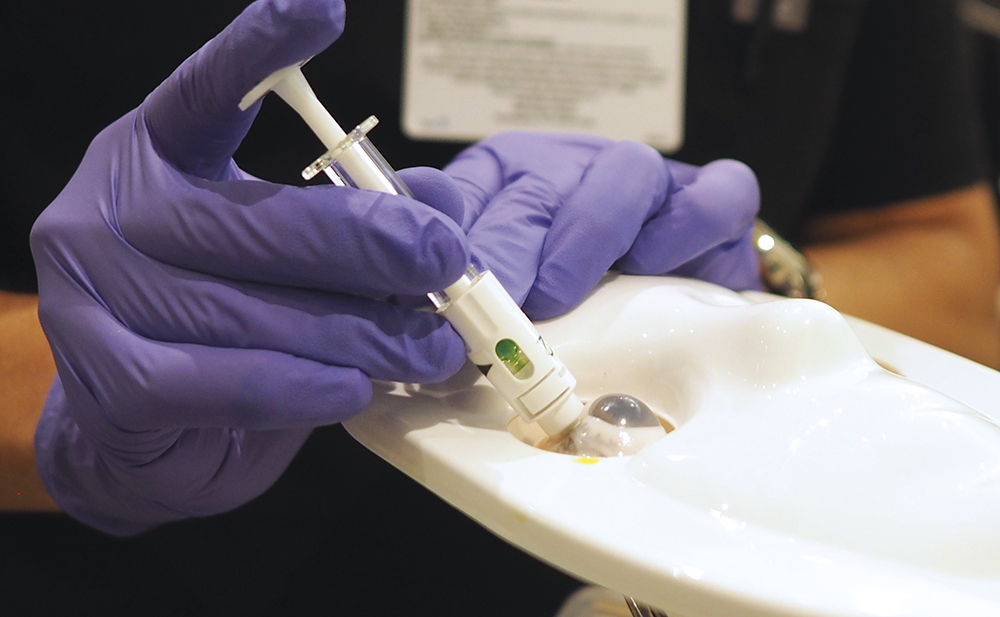
Figure 3: Demonstration of Suprachoroidal Advanceable Microneedle Device’s dial rotation feature to advance the needle while performing injection. Prototype design not available for sale or approved for use in any jurisdiction.
In December 2024, West conducted a directional study with 13 participants, including retina consultants and trainees with varying levels of experience (Figure 4). The purpose of the study was to test the device’s efficiency in performing suprachoroidal injections in terms of precision and accuracy and to assess whether the needle advancement feature can improve the success rate of injections when the free length of the needle is insufficient to access the suprachoroidal space. No formal training was conducted for the users.
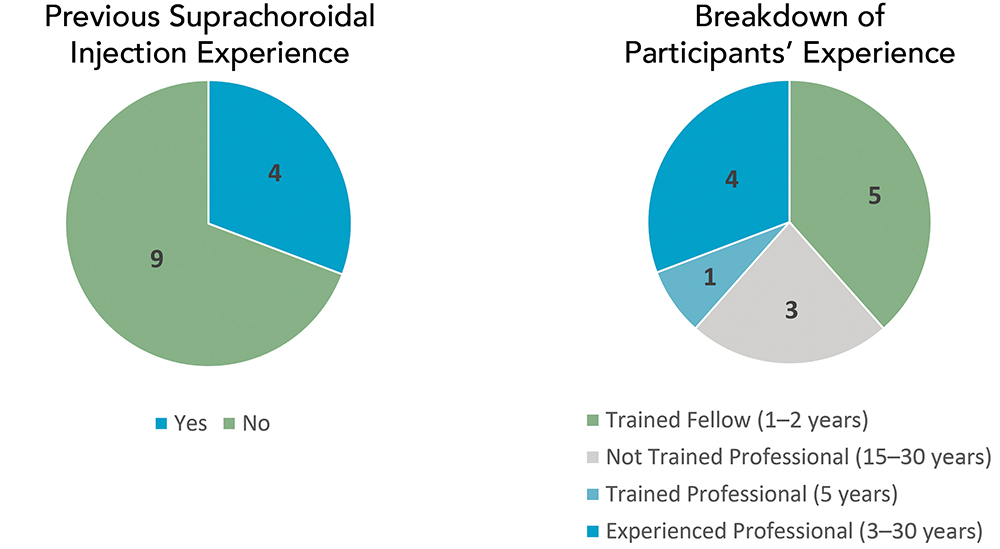
Figure 4: Distribution of participants involved in the early directional study.
Fresh porcine eyeballs (used within 48 hours post-euthanasia) were used for this study and were optimised to standard intraocular pressure using normal saline injections prior to the tests. Each participant performed four suprachoroidal injections in independent porcine eyeball samples, after which the success of the injection and the use of the advancement feature was assessed. The successful injections were determined based on the gross dissection of the porcine tissue to qualitatively assess the expansion of the fluorescent dye in the suprachoroidal space (Figure 5).
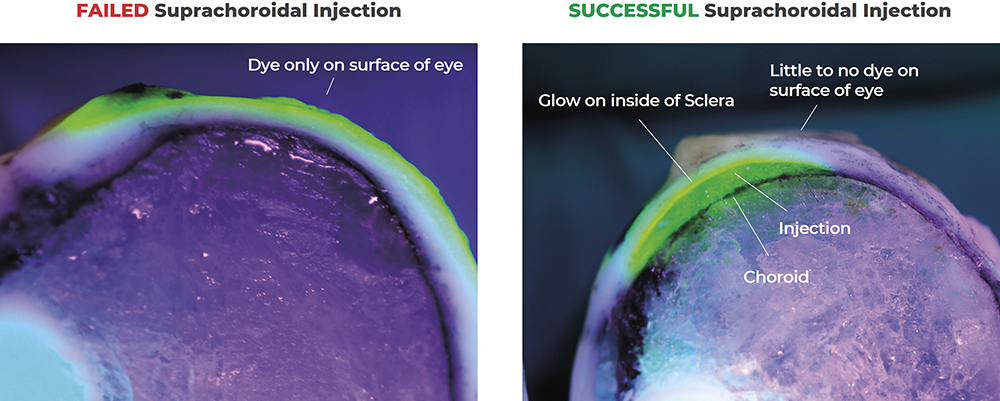
Figure 5: Demonstration of a failed versus successful suprachoroidal injection. In successful suprachoroidal injections, the potential space between the sclera and the choroid is seen expanded with the fluorescent drug surrogate, whereas in failed injections the dye is only seen on the surface of the sclera.
As seen in Figure 6A, the participants with experience injecting into the suprachoroidal space had a success rate of 100%. Within these successful injections, 75% were performed without using the extension feature, while 25% used the extension feature – a number consistent with the need seen in clinical cases where 25–30% of patients may have thicker sclera, necessitating the use of a longer needle.6
For inexperienced participants, the trained fellows with less than one year of retina experience were eliminated from the assessment, after which the success rate was 44%. The advancement feature was used successfully in 57% of the injections. Although extensions were applied more in failed injections than in successful ones, this was due to the lack of applied pressure, as inexperienced participants seemed unfamiliar with the baseline pressure and dimpling needed to conduct a suprachoroidal injection. Breaking down the results further, the inexperienced professionals showed failure even without extension in four cases, which was likely due to the lack of applied pressure in the injection (Figure 6B).
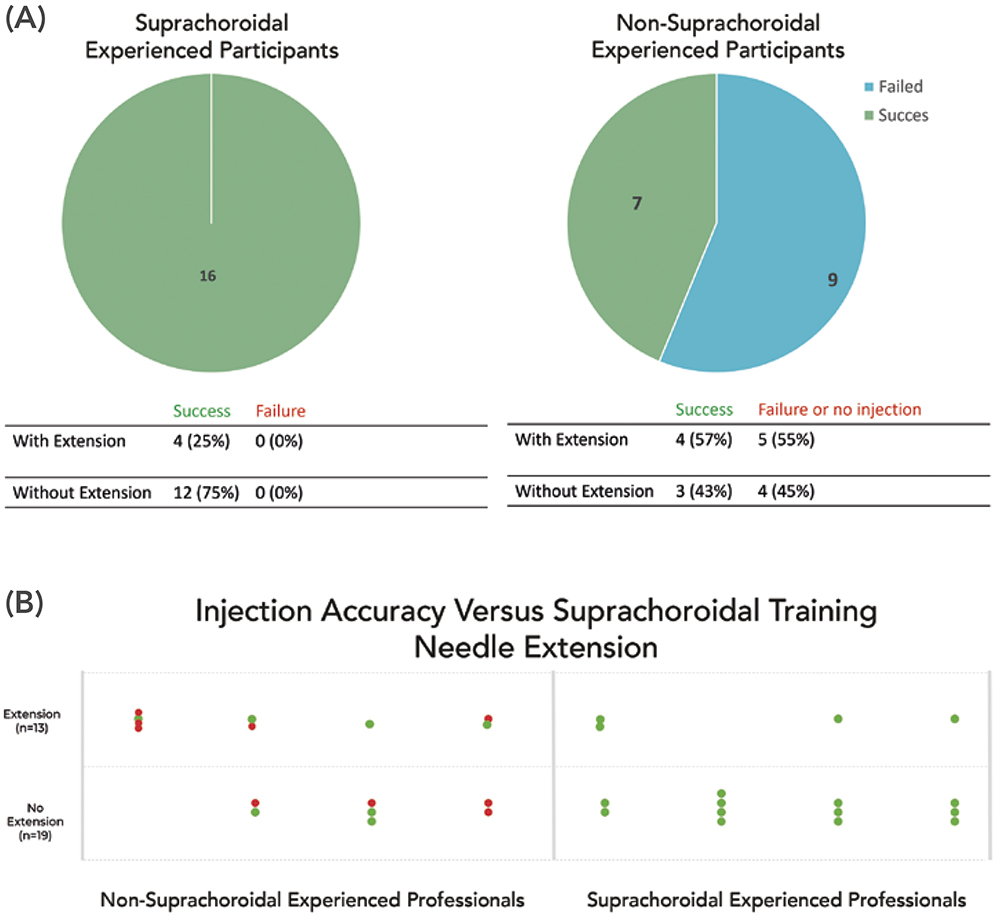
Figure 6: Success rate of suprachoroidal injection with participants who are experienced and inexperienced with suprachoroidal injections. Breakdown of success
rate with and without extension feature.
SUMMARY AND FUTURE DIRECTIONS
Participants thought that accurately inserting the needle into the suprachoroidal space was primarily dependent on the needle lengths built into the device. West observed that needle depth accuracy was also highly dependent on the force applied by the user and other techniques, such as twisting and pressing the needle into the eye.
Healthcare professionals with experience performing suprachoroidal injections knew that dimpling is critical for a higher success rate, which was reflected in the success rate of injections performed by this group. In thicker sclera, the extension feature was helpful in performing a successful injection, albeit only when constant pressure was applied to the surface of the eye. A goal for future research is to focus on device-dependent techniques that minimise injection time and discomfort, optimising both patient experience and procedural efficiency.
The suprachoroidal approach demands mastery of new techniques and collaboration between drug delivery device engineers, pharma companies and the retina community. Improving the comfort and timeliness of suprachoroidal injections is critical.
COLLABORATION AND ONGOING RESEARCH
Collaboration with pharmaceutical and ophthalmic device partners is crucial to advance the design and adoption of suprachoroidal delivery systems. West’s expertise in container closure integrity provides valuable insights into clean, low-particulate packaging solutions with drug packaging solutions for low-volume injections, including for suprachoroidal delivery. West invites collaborations for drug-product specific optimisation and development of the Suprachoroidal Advanceable Microneedle Device for clinical trials.
ACKNOWLEDGEMENTS
The authors would like to thank Rajiv Kumar, Kevin Marett and Joao Santos for their respective contributions to this programme. The authors would also like to acknowledge Archimedic (PA, US) for its support on directional testing study. Lucentis® is a trademark of Genentech, Inc.
REFERENCES
- Wong WL et al, “Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis”. Lancet Glob Health, 2014, Vol 2(2), pp e106–e116.
- MacCumber MW, “Real-World Injection Intervals in Wet AMD”. Retina Today, May/Jun 2020.
- Wang R et al, “Quantifying burden of intravitreal injections: questionnaire assessment of life impact of treatment by intravitreal injections (QUALITII)”. BMJ Open Ophthalmol, 2022, Vol 7(1), art e001188.
- Cox JT, Eliott D, Sobrin L, “Inflammatory Complications of Intravitreal Anti-VEGF Injections”. J Clin Med, 2021, Vol 10(5), art 981.
- Wykoff CC et al, “Suprachoroidal Space Injection Technique: Expert Panel Guidance”. Retina, 2024, Vol 44(6), pp 939–949.
- Wan C-R et al, “Clinical Characterization of Suprachoroidal Injection Procedure Utilizing a Microinjector across Three Retinal Disorders”. Transl VisSci Technol, 2020, Vol 9(11) art 27.

