To Issue 156
Citation: Primavessy D, Saaler-Reinhardt S, Maksymowicz K, Stockton M, “A Novel Instrument to Streamline Combination Product Development”. ONdrugDelivery, Issue 156 (Jan 2024), pp 97–100.
Daniel Primavessy, Sigrid Saaler-Reinhardt, Katarzyna Maksymowicz and Marie Stockton introduce the Gx Inbeneo® platform simulator. This tool supports pharmaceutical companies in bridging pharmaceutical formulation and medical device development processes, reducing time to market for patient-centric combination products.
Drug and device development are two disciplines that require specialised skills. The timelines and development processes involved are also distinct and subject to different regulations. This has led to separate departments being established in most pharmaceutical companies, which presents a challenge when bringing a final drug-device combination to market.
“The sensitive nature of biologics and the prerequisite for parenteral administration poses challenges for drug formulation and delivery.”
Drug development is complex and costly, requiring 10–15 years to introduce a drug product to the market. As pharmaceutical formulation employs physical chemistry, the unpredictability of molecular reactions in different concentrations necessitates an experimental and iterative approach. Formulation, volume and concentration adjustments are therefore made up to Phase II clinical studies where dose finding is conducted. Moreover, as the priority for formulation scientists is establishing suitable pharmacokinetics to ensure therapeutic efficacy and drug safety, the parameters of the device are often a secondary consideration. The definition of an appropriate delivery device, therefore, usually only begins shortly before clinical Phase III.
Device development, in contrast, is much quicker and more deterministic – but still takes several years from initial specification to market launch of a final combination product. If device experts can only begin their task once a formulation is fixed, they have the challenge of developing a solution that effectively delivers a predefined formulation, while also optimising the patient experience. This delay places pressure on the department and potentially impacts time to market.
IMPACT OF THE HOMECARE TREND ON FORMULATION DEVELOPMENT OF BIOLOGIC DRUGS
Biologic drugs are becoming increasingly popular due to their effectiveness in treating a range of diseases. However, the sensitive nature of biologics and the need for parenteral administration poses challenges for drug formulation and delivery. The traditional approach is intravenous (IV) administration in a clinical setting, but, while this is effective, it is also time consuming and can become burdensome for patients if they need to undergo frequent treatments.1 Self-administration provides patients with greater independence and reduces the impact on healthcare systems. However, intramuscular and subcutaneous(SC) administration are the only options for independent patient use. The transition from IV administration to SC self-administration has a significant impact on formulation development.2
“The Gx Inbeneo® platform simulator mimics the Gx Inbeneo® platform, enabling different combinations of spring force, needle gauges and volume to be tested with a specific drug product.”
With SC self-administration, the drug dose cannot be easily adjusted to body weight, which means that one-size-fits-all doses usually need to be developed, yet the pharmacokinetic profile of the drug product must facilitate a comparable therapeutic effect. Compared with IV infusions, injection volumes for SC self administration are limited and, therefore, concentrations are usually higher.3 Higher drug viscosity is a challenge for delivery devices, as it usually results in longer injection times or a larger needle, which can be uncomfortable for the patient. Thus, a delivery device needs to balance these two aspects for an optimal patient experience.
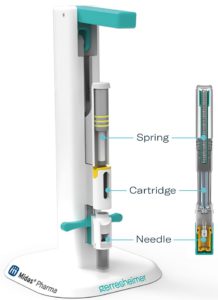
Figure 1: The design of the Gx Inbeneo® platform simulator
directly correlates to the Gx Inbeneo® autoinjector platform
with exchangeable spring, cartridge and needle.
INTEGRATION OF THE GX INBENEO® DEVICE INTO FORMULATION DEVELOPMENT
To expedite time to market for a drug-device combination, and ensure that it supports patient comfort, it is beneficial to test how formulation adjustments impact specific device set-ups earlier than clinical Phase III. However, creating multiple prototypes is time consuming, complex and prohibitively expensive. This is why Gerresheimer, together with Midas Pharma, has developed the Gx Inbeneo® platform simulator, which simulates the Gx Inbeneo® autoinjector platform (Figure 1).
The Gx Inbeneo® is a cartridge-based, single-use autoinjector platform for SC self-administration. It features a patented pre-pressurised design and up to 3 mL fill volume. In contrast to some prefilled syringe systems with a staked-in needle, with the Gx Inbeneo® it is possible to select an appropriate spring and needle for a specific drug product. Injection time can therefore be adjusted to achieve the optimal patient experience.
The Gx Inbeneo® platform simulator mimics the Gx Inbeneo® platform, enabling different combinations of spring force, needle gauges and volume to be tested with a specific drug product. It thus enables practical evidence to be gathered, rather than having to rely on theoretical calculations based on Hagen-Poiseuille’s law. Formulation scientists can therefore quickly assess how adjustments to concentration, volume and viscosity impact device configuration and injection times at any stage of the drug development process. After a test, the expelled drug can subsequently be analysed to ensure that there has been no impact on the drug compound.
As well as being a useful tool in earlier formulation stages, the Gx Inbeneo® platform simulator also aids definition of Gx Inbeneo® prototype configurations ready for clinical testing. The simulator can thus help reduce time pressure on the device department and limit the risk of delays or unforeseen issues far in advance of clinical Phase III.
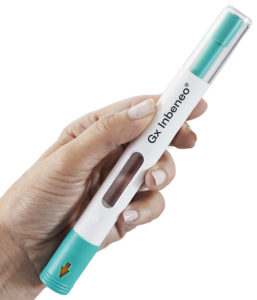
Figure 2: The Gx Inbeneo® is a patient-centric autoinjector for self-administration of biologics.
ENABLING CONFIGURATION TESTING
The Gx Inbeneo® platform simulator enables different configurations of the Gx Inbeneo® autoinjector platform to be tested. The Gx Inbeneo® is intended for the safe, patient-centric delivery of sensitive biopharmaceuticals with a viscosity of up to 100 cP and in volumes of up to 3 mL1 (Figure 2). To achieve this, the autoinjector has a cartridge-based, pre-pressurised design. This innovation employs a robust prefilled glass cartridge to retain the spring force, thus making the primary packaging the force barrier. It also features a double-ended needle that is separated from the primary container until use, which prevents clogging during storage. The needle has a different thickness at each end, with the thicker end of the needle piercing the septum of the primary container, while the end of the needle that pierces the patient’s skin can be thinner. Higher viscosities can therefore be delivered faster than with a standard needle – enabling injection time to be balanced with patient comfort.
The Gx Inbeneo® platform has three variables: cartridge, patient needle gauge and spring force. The device accommodates both 3 mL and 1.5 mL ISO cartridges, needles of 25G, 27G or 29G at the patient end and springs of different forces. The Gx Inbeneo® platform simulator is supplied with all these options to enable different configurations to be tested. Springs are supplied within power packs, each with a different force.
“Due to its quick and simple set-up, it is an extremely efficient tool in the early stages of drug development and throughout formulation adjustments, allowing for swift estimations of a formulation’s behaviour in a final device configuration.”
Once the cartridge is filled with the drug formulation and placed into the simulator, along with chosen needles and springs, it can be manually activated. The needle then pierces the cartridge septum and the formulation is expelled through the needle into a vial. The duration of the simulated injection can be measured and the expelled drug product can be retrieved from the vial for further analysis.
EXPERIMENTAL DEMONSTRATION OF THE PLATFORM SIMULATOR
The capabilities and outcomes of various combinations were tested during three experiments. All data points shown are arithmetic means of quintuplets. Standard deviations were calculated from unbiased variance. The results highlighted the simulator’s versatility in handling formulations with different viscosities and provided insights into potential correlations. The experiments took less than two hours to conduct, which effectively demonstrates the benefits of the Gx Inbeneo® platform simulator for making quick estimations of the behaviour of a formulation in combination with specific device parameters.
Experiment One
In the first experiment, a 30 cP glycerol-water solution was tested with the Gx Inbeneo® platform simulator (Figure 3). Firstly, using a 27G needle, injection times for three different spring forces were evaluated. As shown in Figure 3, an injection time of more than 50 seconds was observed for a low force with a 27G needle. The injection time for the liquid was then tested at a high pressure of 90 N with 25G and 29G needles. With a lower gauge needle, the injection time was improved. However, to best balance delivery time and needle size for optimal patient comfort, a medium or high force with a 27G needle would be optimal for this viscosity.
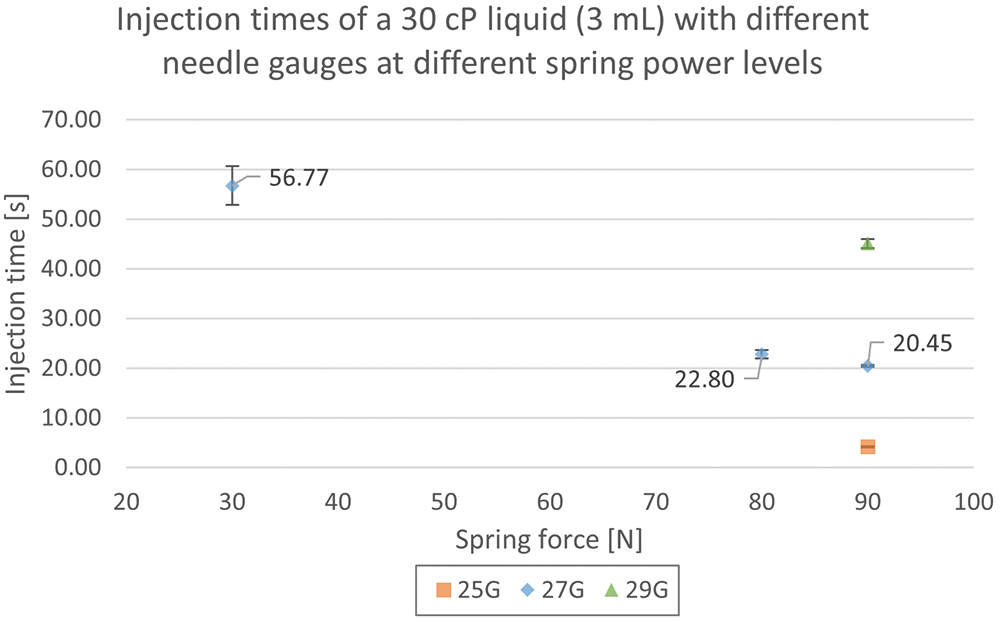
Figure 3: Injection times for 3 mL of 30 cP glycerol solution with three different needles and at different force levels using the Gx Inbeneo® platform simulator.
Experiment Two
Figure 4 shows injection times of water, which has a viscosity of 1 cP, expelled with three different spring forces through a 29G needle. A high spring force of 90 N enabled an injection of 3 mL in <6 seconds. As this is extremely quick, a lower force may be advisable for a viscosity of 1 cP. Therefore, a force of 30 N was also tested, which still resulted in a favourable injection time of <11 seconds, representing a volume flow of 272 μL per second.
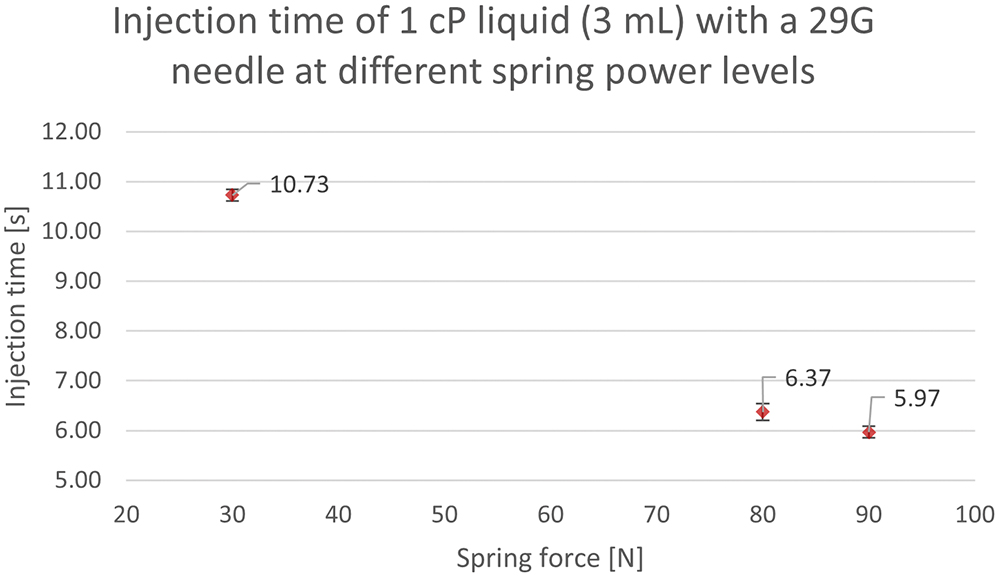
Figure 4: Injection times of 3 mL of 1 cP liquid (water) at three different force levels using the Gx Inbeneo® platform simulator.
Experiment Three
To test the range of the autoinjector, a high viscosity benchmark of 2 mL of 200 cP glycerol solution was expelled with a 90 N spring force and a 25G needle. The liquid could be expelled with an injection time of 18.1 ± 0.21 seconds. This can still be considered an acceptable balance of injection time and needle gauge for the patient, given the volume and extremely high viscosity. At lower volumes, even higher viscosities may be suitable.
CONCLUSION
The Gx Inbeneo® platform simulator can be a valuable instrument for pharmaceutical companies, bridging the gap between pharmaceutical formulation and medical device development. Due to its quick and simple set-up, it is an extremely efficient tool in the early stages of drug development and throughout formulation adjustments, allowing for swift estimations of a formulation’s behaviour in a final device configuration. This innovative approach can significantly streamline the drug-device integration process and potentially reduce overall timelines for bringing a combination product to market. The experiments with the Gx Inbeneo® platform simulator also demonstrate the benefits of the Gx Inbeneo® autoinjector for achieving fast injection times due to its double-ended needle and patented pre-pressurised design.
REFERENCES
- Primavessy D et al, “Gx Inbeneo® – Developing an Autoinjector that Responds to the Challenges of Biologics”. ONdrugDelivery, Issue 152 (Oct 2023), pp 32–36.
- Turner MR, Balu-lyer SV, “Challenges and Opportunities for the Subcutaneous Delivery of Therapeutic Proteins”. J Pharm Sci, 2018, Vol 107(5), pp 1247–1260.
- Zharkov A et al, “Development pathways for subcutaneous formulations of biologics versus biosimilar development”. Expert Re. Precis Med Drug Dev, 2022, Vol 7(1), pp 62–69.

