Citation: Katz M, Ratigan M, “A Vial-Based Solution for Wearable Drug Delivery. ONdrugDelivery Magazine, Issue 100 (Sep 2019), pp 68-71.
Mindy Katz and Michael Ratigan describe how, due to the inherent challenges of integrating vials into wearable devices, there has not been a viable, patient-centric option for wearable devices using vials … until now.
“Maintaining a vial as the primary drug container can significantly improve time to commercialisation, while reducing both cost and overall risk for pharma companies bringing new biologics to market.”
With the continued shift to value-based care, increased emphasis on patient drug delivery experiences, and rising healthcare costs, pharmaceutical companies are under mounting pressure to offer solutions capable of addressing the varied needs of both patients and healthcare providers. Innovations in the drug delivery and combination product industry have seen standard primary containers incorporated into prefilled and preloaded drug delivery devices, with the intention of creating a patient-centric solution that benefits both healthcare providers and pharma companies.
A recent example is the use of a standard vial as the primary container for wearable drug delivery devices. Maintaining a vial ast he primary drug container can significantly improve time to commercialisation, while reducing both cost and overall risk for pharma companies bringing new biologics to market. However, the complexities involved in using a vial for wearable drug delivery devices have – until now – made such solutions impractical for pharma companies and patients alike.
CHOOSING A PRIMARY CONTAINER
There are numerous considerations drug developers must take into account when selecting a primary container.1 Volume, material, manufacturer, previous regulatory approvals, procurement concerns and costs – as well as any chemical interactions with the specific medication – are all in the mix when it comes to choosing an ideal container closure system (Figure 1). Consequently, one of Sorrel’s initial goals was to allow pharma partners the freedom to use a variety of drug reservoirs. The technology was thus designed with the necessary flexibility to allow drug developers to integrate the primary container of their choice.

Figure 1: Primary containers for injectables: vials and cartridges.
THE BENEFITS OF A VIAL
Over the past decade, the drug delivery industry has seen new primary containers and drug delivery devices introduced to the market, bringing to light innovations, including novel materials and drug containers, for the benefit of both patients and drug manufacturers. Despite this, for the vast majority of injectable medications, drug formulation and development continues to occur in a vial. Compared with prefilled syringes and cartridges, the vial is still the standard and most widely used primary container for injectables.
“Any transition from one primary container to another introduces cost and time needed for activities on all fronts – technical, procurement, supply chain and regulatory, to name but a few.”
Accordingly, vials are being manufactured in larger numbers and with the lowest cost per unit, compared with other primary containers. Additionally, filling lines for vials are the most common, and many contract manufacturing organisations (CMOs) offer vial filling capabilities, as opposed to less-popular cartridge and prefilled syringe filling lines. Many of the large pharma companies have established internal capabilities for handling vials, while needing to outsource the knowledge and filling of other primary containers.
Pharma companies have traditionally encountered the need to transition from one primary container to another ahead of a commercial launch. This generally involves transferring the medication from a vial to either a cartridge or prefilled syringe to allow for a more patient-friendly and ready-to-inject application. However, this transition to a new primary container introduces new challenges and risks to the development project.
First, the addition of any new container closure component – specifically plungers which are integral to both cartridges and prefilled syringes – is another material that comes in contact with the medication and must be vetted and tested accordingly. This may require bringing in an additional manufacturer, and possibly a dedicated development project, to supply a proprietary plunger for the system. Additionally, both prefilled syringes and cartridges generally require siliconisation to support the plunger movement, which can pose a challenge for medications known to interact with silicone.
Regardless of these specific challenges, any transition from one primary container to another introduces cost and time needed for activities on all fronts – technical, procurement, supply chain and regulatory, to name but a few. These activities introduce inherent risks to a development project and may cause delays in launching a new drug product to market. Pharma companies are therefore faced with a challenging decision when launching a new medication – deciding in which primary container, together with an appropriate drug delivery device, their product will be introduced to the market.
WEARABLE DEVICES MEET VIALS
Wearable drug delivery devices (also referred to as patch pumps, wearable injectors, on-body devices, large-volume injectors or bolus injectors) have gained popularity in recent years and can provide a significant value proposition for the delivery of large-volume and high-viscosity medications. Moreover, therapies requiring a more complex treatment profile than the traditional manual injection can benefit from such devices, providing options for timed treatment initiation, alternating flow rates, patient-controlled boluses, paused injections and more. Accordingly, we see many pharma companies partnering with medical device manufacturers, as well as developing in-house capabilities, to support the launch of combination products with wearable injectors in the upcoming years.
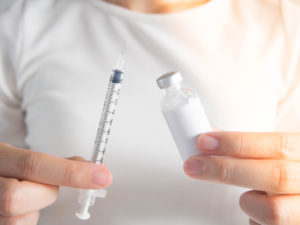
Figure 2: Filling certain wearable devices today requires manually transferring medication from a vial via a syringe.
Looking at such devices, both commercially available and those currently in development or in clinical trials, we see wearable device manufactures recognising the importance for their pharma partners of maintaining the vial as the primary container. Accordingly, there have been several attempts to introduce vial-based wearable drug delivery solutions to the market. However, such solutions generally shift the focus from the patient to the partner. While allowing pharma companies to maintain the vial as the primary container, these generally result in the patient having to perform additional actions in order to transfer the drug out of the vial and into the drug delivery device – affecting the patient centricity of the overall solution.
Patients will often have to purchase the medication and device separately or receive the two components separately but co-packaged. In order to transfer the medication from the vial into the device, one option will be for the user to manually fill the device. This will entail withdrawing the medication from the vial with a dedicated syringe and then transferring it via a dedicated fill hole into the device (Figure 2).
Alternatively, a dedicated transfer accessory is an option for some devices, allowing the medication to be transferred out of the vial and into the wearable device in a semi-automatic manner. Another option, introduced with some wearable devices, is to have an external filling port through which the medication is extracted from the vial and transferred into the device.
While these solutions enable a vial-based wearable drug delivery solution, they also introduce a new set of complexities and challenges for the user. This is especially true for first-time patients, the elderly or those with limited dexterity. In such cases, this may prevent patient adherence to therapy – with the added risk of potential dosing errors.
To date, pharma companies have therefore been left with two options, neither of which are ideal. The first is to introduce a vial-based solution which adds further complexity to the patient experience. The second requires taking on the task of transferring to a new primary container, enabling a more patient-centric approach but introducing additional risk and delaying time to commercialisation.
THE VIAL CHALLENGE
The overriding challenge for a vial-based wearable device derives from the inherent properties of a vial as a non-collapsible drug reservoir. In comparison, a cartridge or prefilled syringe is collapsible, comprising an open-ended glass or plastic barrel with a plunger that moves as the delivery progresses, continuously reducing the overall volume of the reservoir as the drug is expelled.
When examining the different types of drug delivery mechanisms, one can either push or pull the drug out of a primary container to transfer the fluid from the reservoir through the device fluid path and into the patient. Pushing requires exerting a force on the back end of the plunger and so is not a suitable mechanism to be used with a vial.
On the other hand, drawing the medication out of the primary container poses a new challenge. As the drug is drawn from the vial, air pressure inside the vial is reduced and vacuum is created – making it more challenging to withdraw fluid from the vial. As an example, when using a syringe to manually draw directly from a vial, users are instructed to inject air into the vial prior to withdrawing medication – allowing the medication to be withdrawn more easily, and preventing vacuum forming.
Another inherent challenge pertains to the orientation of the vial during the withdrawal of the medication. As the vial is comprised of both air and liquid, in order to withdraw the medication out of the vial, the vial’s orientation needs to be with the cap facing down, relying on gravity for maintaining the flow of medication.
These dual requirements, avoiding creation of vacuum in the vial while maintaining the vial’s correct orientation when drawing out the medication, introduce technical challenges for any vial-based wearable device.
A PLATFORM SOLUTION FOR WEARABLE DRUG DELIVERY
Sorrel’s pumping mechanism, integrated with the platform’s smart sensing capabilities along with audio, visual and tactile indicators, is able to address the challenges of a vial-based wearable device. A key component of the platform solution involves the ability to decouple the device’s pumping mechanism from the primary container, enabling the flexibility to accommodate a wide range of drug reservoirs, cartridges and vials alike.
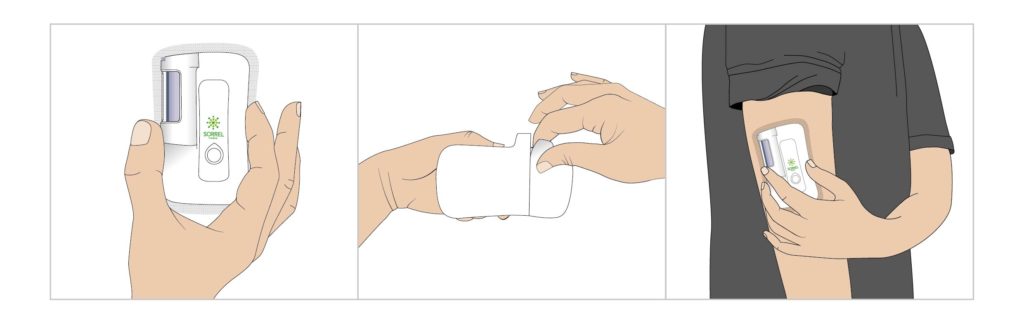
Figure 3: Prefilled and preloaded wearable device user flow: peel and stick.
Having established that a device platform must comprise a truly patient and partner-centric solution,2 it was important to base this solution on Sorrel’s platform, without compromising the ease of use of its prefilled and preloaded wearable devices (Figure 3).

Figure 4: UV-C LED-based disinfection chamber, enabling a prefilled and preloaded device.
As previously noted, Sorrel’s platform solution enables a prefilled and preloaded device configuration, by using a disinfection chamber with an integrated UV-C LED, for disinfection at point of care.3 The LED disinfects the primary container’s septum prior to engagement between the primary container and the rest of the device’s fluid path (Figure 4).
Sorrel’s proprietary UV-C LED technology provides disinfection at the point of care; meets the size, cost and energy requirements of a fully disposable wearable device; and fits both vials and cartridges – thus complementing Sorrel’s vial-based solution.
SUMMARY
Despite the introduction and popularity of new primary container configurations, pharma and biotech companies have nevertheless long held a clear and vested interest in the direct use of vials for drug delivery. Due to the inherent challenges of integrating vials into wearable devices, there has not been a viable, patient-centric option for wearable devices using vials – until now.

Figure 5: Sorrel Medical’s wearable device platform, compatible with both cartridges and vials (pictured: 5, 10 and 20 mL).
The option of using a vial as the primary container, complementary to the cartridge-based device offering, is a strategic new component of Sorrel’s commitment to providing a patient-centric and partner-focused platform solution to the world of drug delivery (Figure 5).
REFERENCES
- Siew A, “Container Selection for Biologic Formulations”. Pharmaceutical Technology, 2016, Vol 40(4), pp 24–26.
- Katz M, “Prefilled wearable drug delivery: harnessing technology for patient & partner centricity”. ONdrugDelivery, Issue 91 (October 2018), pp 32-36.
- Katz M, “UV sterilisation & the case for prefilled &preloaded drug delivery systems”. ONdrugDelivery, Issue 95 (February 2019), pp 80-84.
Previous article
MITIGATING RISK TO DELIVER SUCCESS IN HIGH-VOLUME WEARABLE DEVICESNext article
COMPANY SHOWCASE: Sensile Medical
