To Issue 152
Citation: Tumlinson A, “Acquiring a Liquid Biopsy Incidental to Intravitreal Injection”. ONdrugDelivery, Issue 152 (Oct 2023), pp 76–79.
Alexandre Tumlinson introduces Twenty/Twenty Therapeutics‘ VitreoDx injection system to address emerging trends in intravitreal therapeutics.
NEW INTRAVITREAL THERAPIES HIGHLIGHT UNMET NEED
Any fluid injected into the closed space of the eye results in a short-term spike in intraocular pressure (IOP), which then resolves over the next minutes or hours.1 If the spike is large enough, the patient may lose light perception in the eye until the pressure normalises and adequate perfusion returns. In this case, the doctor will carefully monitor IOP, and may perform paracentesis (i.e. using a 30G needle to relieve excess fluid through the cornea of the eye) if the patient does not recover sight within a few minutes.
“As blockbuster anti-VEGFs lose patent protection, the IVI space is predicted to fill with new entrants promising identical results at lower cost.”
The 50 μL dose typically delivered with traditional anti-vascular endothelial growth factor (anti-VEGF) therapies for wet age-related macular degeneration (AMD) and diabetic retinopathy rarely results in a pressure spike of clinical significance.2 However, two recently approved therapies for geographic atrophy (GA) are both formulated with a 100 μL dose volume; injection volumes of this size result in a larger number of high IOP events, requiring careful pre-injection coaching of patients, and often require post-injection management.3,4 A greater challenge looms, however, as many patients suffer concurrent GA and wet AMD,5 especially as modulation of the complement pathway may increase the incidence of neovascular events.6
High-viscosity, large-molecule biologics increase the time and physician effort required to deliver a therapy through a small gauge needle. One of the GA drugs mentioned previously is recommended for delivery with a 29G or larger needle. However, in clinical trials, the other drug was delivered in two separate doses (at the same appointment) due to its high viscosity.7
Reports of rare, vision-threatening occlusive vasculitis have plagued several recently approved and pipelined therapeutics. Sharma et al (2022) warn that inflammatory effects are not new, but these cases represent a vision-threatening extreme of side effects that are actually common for intravitreal therapies, going on to recommend vigilance in the use of all new intravitreal biologics.8
As blockbuster anti-VEGFs lose patent protection, the intravitreal injection (IVI) space is predicted to fill with new entrants promising identical results at lower cost.9 Biosimilars will drive adoption against an established preference for reference drugs, while established drugs will struggle to defend market share against lower-cost alternatives.10
Future pipelined drugs acting on novel pathways promise benefit to individuals whose disease is driven primarily via those pathways.11 On the other hand, retina care decisions are currently driven by what the specialist can see via structural imaging. While optical coherence tomography, fluorescence angiography and direct or photographic visualisation of the fundus can tell healthcare professionals if a patient has GA or swelling associated with neovascularisation, it cannot tell them the degree to which various pathways contribute to disease in a particular patient. Faced with this lack of information, doctors will generally choose the first line drug that works best for most patients, and then potentially trial an alternative if some criteria of observable change are not observed in response.
“VitreoDx has the potential to mitigate problems currently facing
intravitreal therapy.”
VITREODX ENABLES ROUTINE LIQUID BIOPSY OF THE VITREOUS HUMOUR
Acquiring a liquid biopsy directly with a 25G needle “tap” procedure prior to a normally indicated IVI has been shown to be effective approximately 90% of the time. Twenty/Twenty performed a similar experiment ex vivo using non-vitrectomised human eyes, aged 76–91 years, suggesting that 30G needle taps may perform similarly in this cohort, in which the vitreous is significantly liquefied.

Figure 1: VitreoDx, Twenty/Twenty Therapeutics’ prefilled cartridge injector for acquiring routine fluid biopsy of the vitreous, incidental to IVI.
Following this evidence, Twenty/Twenty designed a device to perform the “tap and inject” procedure with an optimised workflow and minimal trauma to the patient – VitreoDx (Figure 1). The device is a prefilled, disposable, cartridge-based injector, with a novel aspiration mode. The key technical challenges it addresses include long term storage of 50–100 μL evacuated volumes, reliably switching between aspiration and injection modes in a smooth single motion, and easily removing the acquired sample for analysis. Twenty/Twenty also packages the system in an attractive industrial design that should be intuitively familiar to a retinal surgeon. The final surgical workflow (Figure 2) is simple :
- After opening the sterile package, the needle of the device is inserted through the pars plana as per normal
- The surgeon presses a “button” to begin acquiring the sample
- After two seconds, the surgeon slides the “button” forward to initiate delivery of the drug
- After removing the needle from the eye, the surgeon can easily remove the sealed “button” containing the biopsy from the disposable contaminated sharps to be sent for analysis.
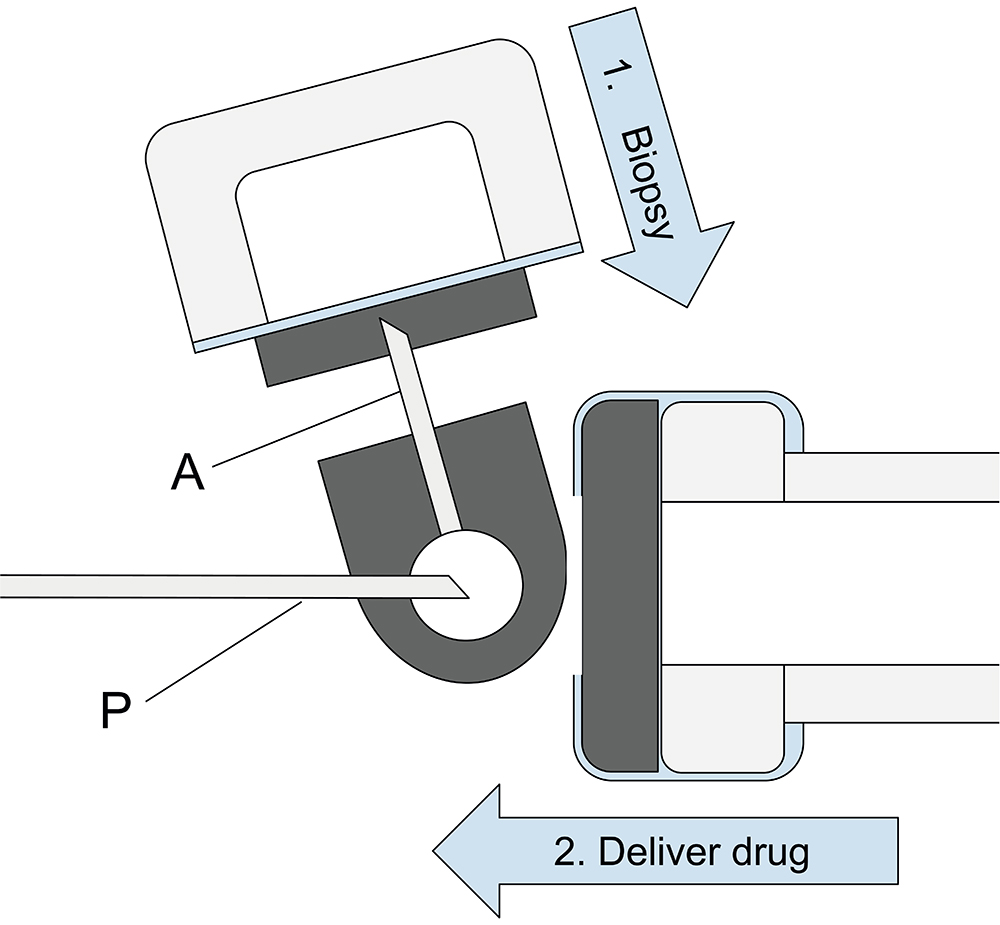
Figure 2: Schematic illustrating VitreoDx’s unique fluidic system for acquiring a neat biopsy of vitreous fluid prior to injecting the drug. Arrows indicate the lateral pressing motion of the button to impale the evacuated biopsy chamber on the accessory needle (A), followed by the axial sliding motion of the button to impale the drug cartridge on the primary needle (P).
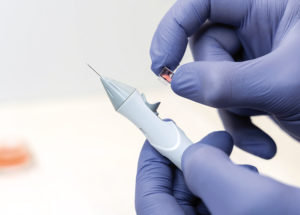
Figure 3: The biopsy (shown with pink dye for clarity) is contained in a self sealing container that can be easily separated from the disposable contaminated sharps after the surgical procedure, and can be sent for laboratory analysis to potentially guide a future treatment.
“In addition to benefits in clinical practice, pharmaceutical developers stand to gain from routine liquid biopsy in research activities.”
Microlitre fluid biopsies from ex vivo human eyes collected with VitreoDx prototypes have been analysed for over a hundred proteins related to angiogenesis and inflammation using a proximity extension assay from Olink (Uppsala, Sweden). Consistent with academic work on surgical vitreous specimens,12 using the same proteomics assays, 90% of the assayed proteins were quantifiable in at least 25% of the submitted samples.
VitreoDx has the potential to mitigate problems currently facing intravitreal therapy. By removing liquid volume prior to injecting drug, VitreoDx prophylactically protects the eye from an IOP spike.13 Because the acquired volume is similar to the injection volume and injection occurs directly after sampling, the eye is only briefly hypotonic, and need not be manipulated as a “soft” eye. When used with paired drugs during the same appointment, VitreoDx is best used with the largest injection, delivered first, to best equilibrate pressure and avoid aspiration of either drug. For a large majority of patients, the necessity of a post-injection paracentesis should be avoided. Because a preloaded spring automatically forces the drug from the cartridge, the surgeon does not lose dexterity struggling to extrude a high-viscosity drug through the needle.
The sample collected by VitreoDx presents a further opportunity for development and value creation (Figure 3). A sample analysis report describing a longitudinal history of the observed protein concentration in known angiogenic and inflammatory pathways, including anti-drug antibodies, can offer the surgeon an alternative viewpoint to supplement structural imaging,12,14 and may aid in appropriately timing the next dose or flagging potential side effects.8 The sample analysis may also help to identify patients who may benefit from highly specific drugs acting on novel pathways, such as by identifying patients who express the drug target at higher than typical levels.
Doctors using this device and analysis may be able to take advantage of procedure codes describing in-office “tap-and-inject” for greater reimbursement. Taken together, the workflow, patient advantages and increased procedure compensation represent an opportunity for a differentiated product, even when packaged with first-line medications that may be pharmacologically similar to competitors.
In addition to benefits in clinical practice, pharmaceutical developers stand to gain from routine liquid biopsy in research activities. For example, clinical trial success is likely to be improved by enriching the study population by selecting subjects who express a drug target protein above a specific threshold.15,16 Companies that engage in routine vitreous liquid biopsy in trials or in clinical practice will have access to large libraries of rich data from which to discover novel drug targets.17
The VitreoDx device described in this article has completed feasibility testing and design for manufacturing is in process. This product in development has not been evaluated by the US FDA and is not available for sale in the United States. Twenty/Twenty Therapeutics is now actively seeking device and pharma partners who are interested in participating in validation activities to discover the value of routine vitreous liquid biopsy.
REFERENCES
- Fuest M et al, “Monitoring intraocular pressure changes after intravitreal Ranibizumab injection using rebound tonometry”. Ophthalmic Physiol Opt, 2014, Vol 34(4), pp 438–444.
- El Chehab et al, “Intraocular Pressure Spikes after Aflibercept Intravitreal Injections”. Ophthalmologica, 2016,Vol 236(1), pp 43–47.
- Toler AR et al, “Is IOP a Concern Post-Injection of Macugen, Avastin, or Lucentis?”. Investig Opthalmol Vis Sci, 2007, Vol 48(13), p 74.
- Lorenz K, Zweiner I, Mirshahi A, “Subconjunctival reflux and need for paracentesis after intravitreal injection of 0.1 ml bevacizumab: comparison between 27-gauge and 30-gauge needle”. Graefes Arch Clin Exp Ophthalmol, 2020, Vol 248(11), pp 1573–1577.
- Kaszubski P et al, “Geographic Atrophy and Choroidal Neovascularization in the Same Eye: A Review”. Ophthalmic Res, 2016, Vol 55(4), pp 185–193.
- Steinle N, Scott A, “Managing Concurrent Neovascular AMD and Geographic Atrophy (GA)”. Modern Retina, Jul 2023.
- Jaff GJ “C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial”. Ophtalmology, 2021, Vol 128(4), pp 576–586.
- Sharma A et al, “Immunogenicity: Clouding the Future of Intravitreal Therapy”. Ocul Immunol Inflamm, 2022, online ahead of print.
- Kapur M, Nirula S, Naik MP, “Future of anti-VEGF: biosimilars and biobetters”. Int J Retina Vitreous, 2022, Vol 8(1), p 2.
- Kolbe AR, “Physician Understanding and Willingness to Prescribe Biosimilars: Findings from a US National Survey”. BioDrugs, 2021, Vol 35(3), pp 363–372.
- Martinez-Alejo JM, Baiza-Duran LM, Quintana-Hau J de D, “Novel therapies for proliferative retinopathies”. Ther Adv Chronic Dis, 2022, Vol 13.
- Lamy R, Stewart JM, “Vitreous Biomarkers: What They Are and How They May Be Used to Advance the Management of Diabetic Retinopathy” in “Biomarkers in Diabetes” (Patel VB, Preedy VR, eds). Springer, Cham, 2022, pp 963–990.
- Enders P et al, “Retinal Nerve Fiber Loss in Anti-VEGF Therapy for Age-Related Macular Degeneration Can Be Decreased by Anterior Chamber Paracentesis”. Ophthalmologica, 2017, Vol 237(2), pp 111–118.
- Micera A, “Biomarkers of Neurodegeneration and Precision Therapy in Retinal Disease”. Front Pharmacol, 2021, Vol 11, Article 601647.
- Nordvang E, “Using Protein Biomarkers Increases the Chances of Success in Clinical Trials”. Proteomics & Metabolomics, Jan 2020.
- Wong CH, Shia KW, Lo AW, “Estimation of clinical trial success rates and related parameters”. Biostatistics, 2019, Vol 20(2), pp 273–286.
- Weber SR, “A validated analysis pipeline for mass spectrometry-based vitreous proteomics: new insights into proliferative diabetic retinopathy”. Clin Proteomics, 2021, Vol 18(1), p 28.

