To Issue 147
Citation: Gross C, Tunkel M, “Addressing Peptide Therapeutics with a Versatile Pen Platform.” ONdrugDelivery, Issue 147 (May 2023), pp 21–24.
Cécile Gross and Mark Tunkel discuss the rising demand in peptide therapeutics and how Nemera’s PenVario pen injector platform, coupled with its end-to-end service offering, has been designed to help pharma partners achieve success in this sector.
The synthesis of the first peptide therapeutic, insulin, occurred more than 100 years ago,1 targeting diabetes mellitus. Ever since, insulin has been widely used to treat this chronic condition. To this day, pharma companies are still working on formulations to improve bioavailability, long-lasting efficacy and patient adherence.
“Since the first US FDA-approved GLP-1 in 2009, pharma companies have worked to reduce injection frequency, improve delivery devices and even offer alternative administration routes, such as oral delivery.”
Considering Type 2 diabetes, for over a decade, another area of interest has been the incretin system.2 Two hormones have been identified – glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide, also known as gastric inhibitory polypeptide (GIP). Both of these hormones are rapidly broken down by the enzyme dipeptidyl peptidase-4 (DPP-4), which encourages the development of degradation-resistant GLP-1-receptor agonists and DPP-4 inhibitors.3 Adding this family of agents to the repertoire of therapeutic blood glucose control has broadened the target patient population from diabetes to obesity. Since the first US FDA-approved GLP-1 in 2009, pharma companies have worked to reduce injection frequency, improve delivery devices and even offer alternative administration routes, such as oral delivery.
In an overview of the evolution of these diseases, in 2019, the International Diabetes Federation estimated that 463 million people between the ages of 20 and 79 are suffering from diabetes, of which 231.9 million are undiagnosed.4 The prevalence of diabetes is projected to reach almost 11% of the global population by 2045. As for obesity, in 2022, the WHO estimated that more than one billion people in the world are obese, including 650 million adults.5 In the US, the prevalence of obesity in over-20s is already more than 40%, according to the National Institute of Diabetes and Digestive and Kidney Diseases.6
A DISPOSABLE PEN PLATFORM TO MEET THE DEMAND IN PEPTIDE THERAPEUTICS
In line with this growing demand and leveraging its long-standing expertise in insulin pens and GLP-1 autoinjectors, Nemera chose to develop a pen injector platform versatile enough to address the aforementioned drugs, as well as others. Now that more than 80 peptide drugs are on the market,7 with several more in clinical trials, covering therapy areas such as migraine, oncology, dry eye syndrome, cardiovascular diseases and even growth disorder, to name but a few, adding a disposable, variable dose device to the already existing pen range seemed obvious.
PenVario is a manual pen platform able to address diabetes and obesity with rapid-acting or long-acting insulins and GLP-1, osteoporosis with parathyroid hormones (PTHs), such as abaloparatide, and fertility with follicle-stimulating hormones (FSHs), such as follitropin alpha. Given the differences between these target populations and regimens, each variant of the platform has been designed to match the specificities of each therapeutic area (Figure 1).
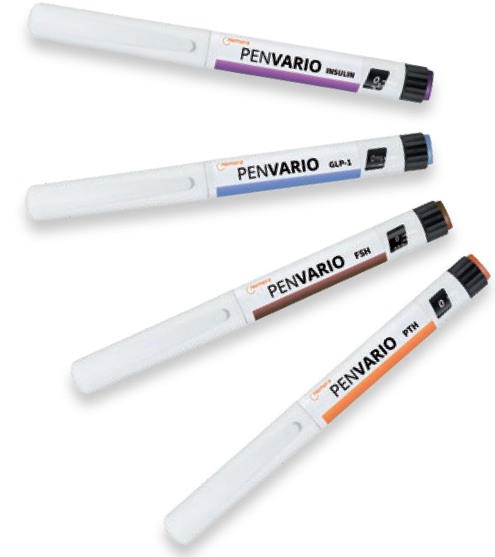
Figure 1: PenVario disposable variable dose pen for multidose therapies, including insulin, FSH, GLP-1 and PTH.
“PenVario is a manual pen platform able to address diabetes and obesity with rapid-acting or long-acting insulins and GLP-1, osteoporosis with PTHs, such as abaloparatide, and fertility with
FSHs, such as follitropin alpha.”
Case Study: Fertility
Although this therapeutic area receives less media attention than diabetes or, more recently, obesity, due to the controversy related to off-label use, infertility does affect a large number of people. According to a recently published WHO report,8 its prevalence is growing, with it estimated to affect up to 17.5% of the adult population worldwide, including both women and men.
There are a few therapeutic solutions and several drugs already available for women or men suffering from infertility, mostly targeting patients between 20 and 45 years old. Ovarian stimulation is sometimes prescribed along with assisted reproductive technologies for women and spermatogenesis for men – two examples of treatments for which an injection of FSH can be required. The regimen usually implies daily injections over the course of several weeks.
Given the characteristics of such a regimen, its duration and potential psychological considerations, the delivery device needs to be as user-friendly as possible to avoid incorrect dosing. Consequently, the FSH variant of the PenVario offers a clearly visible indication of the dosage in milligrams and comes in different versions to accommodate different drug concentrations. Cap fitting and removal forces have also been fine-tuned according to this specific target population, leveraging Nemera’s own human factors capabilities through an ergonomic evaluation.
INTEGRATED SERIVICES AND MANUFACTURING CAPABILITIES
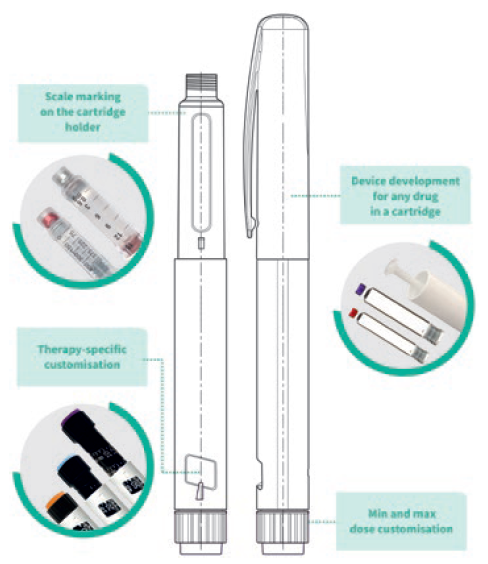
Figure 2: PenVario platform capabilities, tailored to customers’ needs.
For specific customer applications, Nemera can support PenVario with the human factors and patient experience activities that must be integrated to ensure an efficient development process. This ensures that the device, in combination with the drug, is appropriate, safe and effective for the target patient population. This also extends to optimising the patient experience to create competitive differentiation and ensure adherence and engagement with patients and clinical stakeholders.
Customers need to be sure that the device addresses the defined user populations. To this end, early use-related risk analysis activities can help define the human factors and usability programme. Clinical risks must be identified by conducting formative and summative usability testing for all aspects of the device and any supporting assets in alignment with the human factors programme definition, including the production of human factors engineering report documentation for use in for regulatory submissions. This process is linked to developing instructions for use, value-added packaging and digital health related add-ons to support patient engagement and adherence, as well as to extend the value of a device platform (Figure 2).
“Nemera has recently invested into a new manufacturing plant, with the capability to produce prototypes, small series for clinical batches and large-scale automated volumes.”
Nemera can also support regulatory strategy development and support the entire process of developing materials for submission to regulatory authorities, as well as provide laboratory and analytical services. This can also be augmented by fit-for-purpose preclinical, clinical and small-series device supply to accelerate development timelines and defer capital expenses. It is crucial that this is all completed holistically.

Figure 3: Nemera’s brand new state-of-the-art manufacturing facility in Poland.
Furthermore, Nemera is willing to provide a fully automated industrial line to its partners. Nemera has recently invested in a new manufacturing plant (Figure 3), with the capability to produce prototypes, small series for clinical batches and large-scale automated volumes. Sporting state-of-the-art equipment, from moulding to assembly and quality control testing, this brand-new facility includes an ISO 8 cleanroom and complies with BREEAM recommendations (Figure 4); for example, heat is recovered from the process line, and the facility also segregates and sorts all waste, aiming to achieve a 100% recycling rate.

Figure 4: ISO 8 cleanroom compliant with BREEAM
recommendations.
Apart from its end-to-end offering, Nemera is actively working not only with peptides and pen injectors but more globally towards holistic patient-centric solutions. In this regard, its latest move has been to join the Subcutaneous Drug Delivery and Development Consortium to be more involved in this peer-to-peer collaborative hub reflecting on how to address unmet needs.
BENEFITS OF PARTNERING WITH AN INTEGRATED PRODUCT AND SERVICE PARTNER
Nemera’s pen platforms, integrated development, consulting and manufacturing services allow customers to achieve a successful regulatory submission and commercial launch of safe, effective and differentiated combination products with a single partner, applying an agile process across the device and combination product value chain. This will drive patient-centricity, reduction of risk and increased speed to market. This approach can be applied to Nemera’s device platforms or with organic development and allow customers to focus on their core business.
REFERENCES
- Wang L et al, “Therapeutic peptides: current applications and future directions”. Sig Transduct Ther, 2022, Vol 7, Article 48.
- Hinnen D, “Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes”. Diabetes Spectr, 2017, Vol 30(3), pp 202–210.
- Baggio LL, Drucker DJ, “Biology of incretins: GLP-1 and GIP”. Gastroenterology, 2007, Vol 132(6), pp 2131–2157.
- Buchholz K, “We should be worried about global diabetes rates. This chart shows why”. World Economic Forum, Nov 2021.
- “World Obesity Day 2022 – Accelerating action to stop obesity”. WHO, Mar 2033.
- “Overweight & Obesity Statistics”. NIH webpage, accessed May 2023.
- Muttenthaler M et al, “Trends in peptide drug discovery”. Nat Rev Drug Discov, 2021, Vol 20, pp 309–325.
- “Infertility Prevalence Estimates, 1990–2021”. WHO report, Apr 2023.



