To Issue 158
Citation: Bräunlich G, Hahn M, “Deep Breaths, Deep Dives: The New eFlow Integrated Nebuliser”. ONdrugDelivery, Issue 158 (Apr 2024), pp 16–19.
Gesa Bräunlich and Michael Hahn discuss PARI’s new eFlow integrated nebuliser.
With so many different nebulisers on the market – and with numerous highly advanced technological features to consider and even more devices appearing on the horizon – how do you make the right decision to meet the different needs of all stakeholders?
It is important to get this decision right. And yet, for pharmaceutical and biotech companies looking for a suitable delivery solution for an innovative drug formulation, the decision-making process has never been more complex. As Jones & Veasey (2023) have reminded us, “The benefits of getting it right can be enormous, and the consequences of getting it wrong can be dire.”1
It is a difficult balancing act. We value proven and reliable technology to help reduce the risks of clinical development. But we must also look to new technologies to help improve clinical performance, tackle existing limitations, enhance the user experience and ensure future-proof capability, even after many years of clinical development.
“We must look to new technologies to help improve clinical performance, tackle existing limitations, enhance the user experience and ensure future-proof capability.”
When it comes to next-generation delivery solutions, PARI believes it is best to focus on three fundamental elements:
- Optimise the efficiency of drug delivery because many drug formulations are extremely costly
- Enhance usability to help improve the overall patient experience and contribute to enhanced adherence
- Develop smartphone apps to complement smart devices – as in many other industries, this has considerable potential to support patients and inhalation therapies in multiple ways while generating helpful data-based insights.
DRUG DELIVERY EFFICIENCY
Typically, for nebulisers, the higher the volume to reach the lung and the lower the delivered dose, the longer the nebulisation time. The laws of physics cannot be changed. But, to ensure efficiency of nebuliser devices, three separate but interacting parameters can be optimised:
- Delivered dose
- Nebulisation time
- Breathing pattern.
HOW CAN WE INCREASE THE DELIVERED DOSE?
A common approach to increase the delivered dose is to add a breath-trigger function. Breath-triggered nebulisers have been available for around 25 years.2 These types of nebulisers produce aerosols only during – in fact, as part of – the inspiration phase of the breathing cycle. This results in minimised aerosol losses, specifically during the exhalation phase and, therefore, makes efficient use of medication in the nebuliser by delivering high amounts of drug to the patient.3
Cost of goods for innovative drug products, such as antibodies, peptides and nucleotides in the form of antisense oligonucleotides, siRNA or mRNA, can be high. The efficient use of such precious drug products is therefore essential. A further consideration is that toxicology assessments are typically carried out based on the nominal filled dose.4,5 So, in situations where the amount of drug delivered to the patient is not efficacious and the filled dose cannot be increased due to toxicity concerns, a nebuliser with a higher delivered dose may result in an effective dose while not exceeding the maximum allowable clinical dose.
To maximise the delivered dose, PARI has developed a new breath-triggered nebuliser platform based on the proven eFlow technology with its actively vibrating laser-drilled membrane. The applied trigger algorithm enables delivered dose rates of up to 95%.
“It is a balancing act to find the ideal compromise between optimal reduction of inspiratory flow and a convenient breathing mode which is tolerable for the patient.”
WHAT ABOUT NEBULISATION TIME?
Due to the nature of breath-triggered devices, aerosol is only produced during part of the breathing cycle, resulting in relatively long treatment times. In particular, for patients who suffer from chronic conditions, long treatment times increase the burden and need to be reduced to improve the acceptability of therapy.6 An optimal nebuliser delivers drug product with high efficiency – i.e. integrates a high delivered dose and a short nebulisation time.
Achieving short nebulisation times with breath-triggered nebulisers is a universal challenge for which PARI has discovered a solution by taking two approaches:
- Since aerosol is only produced during inhalation, it is necessary to make best use of this short period of time and deliver as much aerosol as possible. This can be achieved by increasing the output rate. Significant improvements made by PARI in the laser drilling process allow aerosol output rates in the range of 1 to 2 mL/min (measured under constant flow conditions – i.e. without a breath trigger) while the particle size of the aerosol remains substantially below 5 μm.
- Short nebulisation times are achieved through an optimised breathing pattern involving a prolonged inhalation time in which more aerosol can be delivered during each breath. Longer inhalation durations – increasing the inspiration:expiration (I:E) ratio – can be achieved by an inspiratory flow resistance that encourages the patient to breathe more slowly.
INFLUENCING THE BREATHING PATTERN
Finally, it is important to take into account that the breathing pattern also has a profound impact on aerosol distribution and deposition in the lungs, which, in turn, fundamentally influences drug effectiveness and patient outcomes.7,8
For high peripheral lung deposition and short nebulisation times, patients should be guided towards a slow deep inhalation with low flow rates. In contrast, high inspiratory flow rates lead to more turbulence in the central airways, which leads to unintended drug deposition in the upper airways.9,10 Importantly, this can be limited by increasing inspiratory resistance.11
There are two main considerations for defining the right inspiratory resistance. While high resistance will successfully decrease inspiratory flow, it might simultaneously have a negative influence on therapy acceptance and adherence due to inconvenience and breathlessness resulting from the increased work of breathing. This is even more relevant if the expiratory resistance is high. For convenience reasons, patients should be able to inhale and exhale through the nebuliser. Consequently, the exhalation resistance should be as small as possible to allow for passive exhalation. It is a balancing act to find the ideal compromise between optimal reduction of inspiratory flow and a convenient breathing mode that is tolerable for the patient.
With the development of the new eFlow Integrated nebuliser, PARI has implemented an innovative breath-guiding approach and a novel nebuliser platform that supports and guides the patient to inhale slowly and deeply. Three factors have been successfully combined to optimise the breathing pattern accordingly (Figure 1):
- Built-in resistance
- Visual and haptic feedback
- App-based breath-guiding.

Figure 1: eFlow Integrated nebuliser platform.
“With this new digital feature, PARI sends feedback to guide the patient to an optimal breathing pattern.”
First, and most importantly, a unique built-in flow resistance limits the peak inspiratory flow. While flow resistance is higher during inhalation to limit flow rates, it is reduced during exhalation to allow optimal work of breathing and tolerability. This is the result of the specific geometry of an innovative built-in resistance termed fluidic diode. The overall aim is to limit patients’ inspiratory flow, as this helps increase lung deposition and reduce nebulisation time.
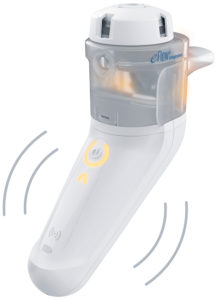
Figure 2: Illuminated nebuliser and
vibrating controller.
The second element to guide patients’ breathing patterns is the user interface on the device. By providing intuitive instructions directly via an illuminated mouthpiece assembly and a vibrating controller, PARI’s new eFlow integrated nebuliser platform effectively trains the patient on the correct breathing pattern during inhalation. A pressure sensor collects real-time data from the inspiratory flow, translating this information into haptic and visual signals that inform the user on whether or not the inhalation is optimal (Figure 2).
Feedback is simultaneously visualised within the new PARI Breath Guide app, which, as an optional feature, is connected to the eFlow Integrated nebuliser via Bluetooth. The visualised breath guiding feature trains a slow, controlled inspiration. The app uses transferred data to provide real-time feedback and a helpful visualisation of inhalation. This can guide the user to inhale slowly and deeply, which, in turn, can increase the I:E ratio and lead to shorter nebulisation times. Such feedback also informs the patient that the inhalation manoeuvre has been performed correctly (Figure 3).
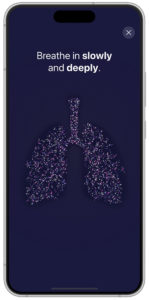
Figure 3: Visualisation of breath guiding in app (prototype).
A more detailed inhalation report is given within the app at the end of each therapy to inform patients about their inhalation technique and therefore strengthen their engagement and self-management. This can support patients in their effective inhalation to promote the best possible therapy outcomes. With this new digital feature, PARI sends feedback to guide the patient to an optimal breathing pattern.
USABILITY – A PATIENT-CENTRED APPROACH
The development of a new nebuliser is highly dependent on patient understanding and patient acceptance. As a result, from the very beginning of development, human factors studies have played a significant role in shaping the entire product concept and continuously improving various device features and the user interface. The learnings from this design process resulted in a user-friendly nebuliser, which includes an integrated controller and which considers the specific characteristics of target users with the ergonomic nebuliser design, the conical grip for different hand dimensions and the secure stand for easy filling of the reservoir. Specific user characteristics were also considered when implementing the user interface of the breath-guiding features (Figure 4).
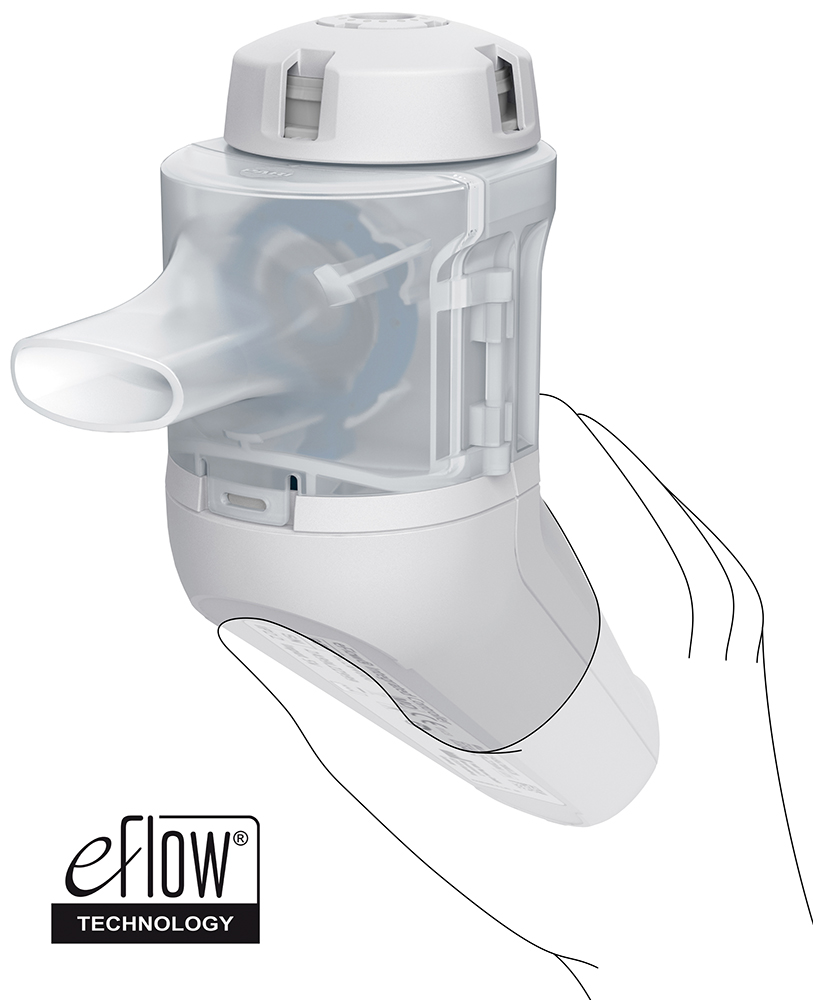
Figure 4: Ergonomic design.
The eFlow integrated nebuliser was tested in several human factors studies in Germany and the US with relevant patient populations, and further studies are planned, always with regulatory requirements in mind. Substantial work and testing were performed to ensure that all visual, haptic and acoustic signals are easily understood by the user. Critically, this forward looking approach will also facilitate the preparation of upcoming registrations of drug-device combination products and shorten lead times for partner-specific configurations.
THERE IS NO NEED TO CHOOSE
The novel nebuliser platform optimises drug delivery with an innovative breath-guiding approach. With its patient-centric approach, PARI has made significant advances in optimising the device platform and the entire patient experience to drive adherence and improve performance and clinical use – factors that can translate into clinical success. Beyond this innovative product design, the well-established eFlow aerosol generator, which is a core component, is produced on proven commercial-scale production equipment in two independent manufacturing sites.
With the new eFlow Integrated nebuliser, PARI has achieved a high level of integration – the successful blend of:
- High delivered doses achieved in short nebulisation times
- An ergonomic and intuitive nebuliser design combined with a useful smartphone app
- Innovation based on the proven eFlow technology.
The new eFlow Integrated nebuliser is still under final development, while it has already confirmed expectations during the first in vitro studies with innovative drug products.
To discover more about eFlow, visit: www.pari.com/int/eflow-technology-partnering.
ACKNOWLEDGEMENTS
Martin Guppy, PhD, participated in the writing and proofreading of the manuscript.
REFERENCES
- Jones M, Veasey R, “Due diligence – the key to success in drug delivery device selection”. ONdrugDelivery, Issue 145 (Apr 2023), pp 20–24.
- Denyer J, Dyche T, “The Adaptive Aerosol Delivery (AAD) technology: Past, present, and future”. J Aerosol Med Pulm Drug Deliv, 2010, Vol 23(Suppl 1), pp S1–10.
- Hardaker LE, Hatley RH, “In vitro characterization of the I-neb Adaptive Aerosol Delivery (AAD) system”. J Aerosol Med Pulm Drug Deliv, 2010, Vol 23(Suppl 1), pp S11–20.
- Forbes B et al, “Challenges in inhaled product development and opportunities for open innovation”. Adv Drug Deliv Rev, 2011, Vol 63(1–2), pp 69–87.
- Tepper JS et al, “Breathe In, Breathe Out, Its Easy: What You Need to Know About Developing Inhaled Drugs”. Int J Toxicol, 2016, Vol 35(4), pp 376–392.
- Dobler CC et al, “Treatment burden should be included in clinical practice guidelines”. BMJ, 2018, Vol 363, p k4065.
- Nikander K et al, “Mode of breathing-tidal or slow and deep-through the I-neb Adaptive Aerosol Delivery (AAD) system affects lung deposition of (99m)Tc-DTPA”. J Aerosol Med Pulm Drug Deliv, 2010, Vol 23(1), pp S37–43.
- Van Velzen AJ et al, “The influence of breathing mode on tobramycin serum levels using the I-neb AAD system in adults with cystic fibrosis.”. J Cyst Fibros, 2015, Vol 14(6), pp 748–754.
- Dolovich MA, “Influence of inspiratory flow rate, particle size, and airway caliber on aerosolized drug delivery to the lung”. Respir Care, 2000, Vol 45(6), pp 597–608.
- Laube BL et al, “Targeting aerosol deposition in patients with cystic fibrosis: effects of alterations in particle size and inspiratory flow rate”. Chest, 2000, Vol 118(4), pp 1069–1076.
- De Boer A et al, “Inhalation characteristics and their effects on in vitro drug delivery from dry powder inhalers Part 1. Inhalation characteristics, work of breathing and volunteers’ preference in dependence of the inhaler resistance”. Int J Pharmaceut, 1995, Vol 130(2), pp 231–244.
Previous article
THE EVOLUTION OF PULMONARY & NASAL DRUG DELIVERYNext article
Interview with Sandy Munro, Vectura
