Citation: Vecellio L, Le Pennec D, Grevin G, Regard A, “Deposition in Three Nasal Cast Models Using RetroNose Concept”. ONdrugDelivery Magazine, Issue 96 (Apr 2019), pp 42-44.
Laurent Vecellio, Déborah Le Pennec, Guillaume Grevin and Alain Regard discuss a study conducted to test particle deposition characteristics of the RetroNose device prototype for nasal drug delivery.
INTRODUCTION
Nasal drug delivery is linked with interest in three fields of pharmaceutical targeting:
- Topical
- Systemic
- Central nervous system.
The clinical efficacy of a nasal treatment depends on how it is deposited in the nose, because the pharmaceutical target (local, systemic, brain) is directly related to a specific nasal anatomical site. A recent study on chronic rhinosinusitis (CRS) patients has shown how the deposition distribution of corticosteroids in the nasal cavities can have an impact on clinical outcomes.1
“This new device, RetroNose, uses a pMDI to administer the drug through the buccal cavity during the nasal expiratory phase.”
This study has demonstrated the importance of homogenous deposition in the different target regions of the nasal cavity for treating CRS. The turbinates, the maxillary sinuses and the ethmoid regions have been identified as important drug delivery target sites for local treatment of inflammation and infection in rhinological pathologies.2 Systemic delivery is enhanced by exposing the drug to the middle and inferior turbinates and the septum which have a high vascularisation and large surface area (around 150 cm2).
An alternate nasal delivery device concept, RetroNose, has been developed to achieve better drug deposition in the different regions of the nose (Figure 1).3-4
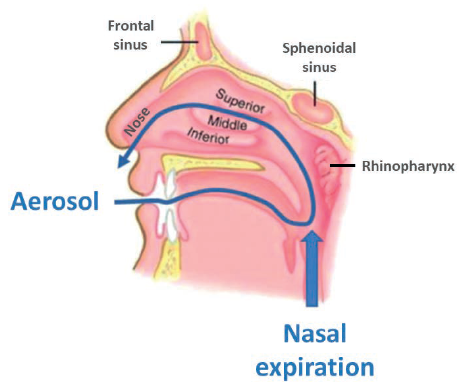
Figure 1: The RetroNose concept: drug delivery through the buccal cavity during the nasal expiratory phase, causes drug particles to enter the nasal cavities through the rhinopharynx.
RetroNose (Figure 2) uses a pressurised metered dose inhaler (pMDI) to administer the drug through the buccal cavity during the nasal expiratory phase. Drug particles enter the nasal cavities through the rhinopharynx, which has a significant impact on drug deposition profile. A recent study has already presented data from five asthmatic patients with rhinosinusitis treated with an aerosol therapy exhaled through the nose5-6 using a similar concept.
The evaluation of nasal drug delivery device performance is traditionally done via regulatory in vitro tests:

Figure 2: RetroNose prototype device examples.
- The dose delivery is measured and gives information about the consistency of dose delivered to the patient.
- The droplet size informs about the particle size distribution and potential lung penetration.
- Spray plume and spray pattern give information about the geometry of the spray.
Although these measurements are relevant for describing the in vitro characteristics produced by a nasal sprays pump, they are not adapted for the
RetroNose device, as the medication is administered orally. Instead, anatomical models such as cadaver heads,7 nasal cavity replicas8 or nasal casts9 are used to measure drug deposition across anatomical regions. Nasal casts are consequently more adapted for performance evaluation of the RetroNose device.
The main objective of the study discussed hereafter was to compare the deposition obtained by the RetroNose prototype with a nasal spray pump using three different nasal casts.
MATERIAL AND METHODS
“A high variability in deposition was observed for the nasal spray compared with the RetroNose prototype device for the different regions of nasal cavities, except for in the middle part.”
The RetroNose prototype device was a pMDI (Inhalia®, Nemera, France) filled with HFA 134a gas (no surfactant) and a 12 μm active compound (API-1) particle size, resulting in a 14.8 ±0.4 μm mass median aerodynamic diameter measured by cascade impactor. A standard nasal pump (Flixonase®, GSK France) was filled with API-1 solution, resulting in a 48 ±2 μm volume mean diameter measured by laser diffraction (n=3).
API-1 deposition in the nasal casts was studied using three different anatomical models, one developed by the Virginia Commonwealth University (VCU, Richmond, VA, US),10 and two 3D-printed models recently obtained from females (FANI 1 & FANI 2, CEPR, INSERM U1100, University of Tours, France). Nasal casts were connected to a mouth and a lung model, including a filter to measure potential drug penetration in the lungs.
Different regions of interest were defined in the nasal casts:
- Mouth
- Nose
- Upper part of the nasal cavity
- Middle part of the nasal cavity
- Lower part of the nasal cavity
- Rhinopharynx.
Three additional regions of interests were added for the FANI 1 & 2 nasal casts (maxillary sinuses, frontal sinuses, sphenoids). The different regions were washed with different volumes of sodium hydroxide using syringes. API-1 was assayed by a spectrophotometric method.
An additional experiment was performed using a second active product (API-2).
A Flixotide®(125 μg fluticasone, GSK France) pMDI was used in the RetroNose prototype and was radiolabelled with Tc99m, as previously described by Chand et al.11 Nasal administration was performed on the VCU model. Deposition was imaged using a gamma camera and was fused with a nasal cast scan.
RESULTS
Results from the experiments demonstrated the following:
- No active compound was detected in the lung model for nasal spray and RetroNose.
- About 50% of the drug was deposited in the nose for the nasal spray.
- About 50% of the drug was deposited into the mouth using the RetroNose prototype.
- Less than 5% of the delivered dose was exhaled in ambient air using the RetroNose device.
- Results showed a major deposition in the middle part of the nasal cavity using the RetroNose device, in contrast to the nasal spray which emphasised deposition in the front of the nose.
- Greater depositions in maxillary sinuses, sphenoides and frontal sinuses were detected when using the RetroNose device compared with the nasal spray.
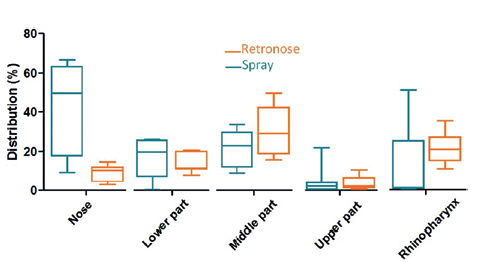
Figure 3: Drug deposition in the different regions of the nasal cavities (upper airway model) using three different nasal cast models for a standard nasal spray (A) and RetroNose (B). (Results expressed in terms of deposited fraction in nasal cavity, n=3 for each nasal cast.)
A high variability in deposition was observed for the nasal spray compared with the RetroNose prototype device for the different regions of nasal cavities, except for in the middle part (Figure 3). This variability should be more important for a nasal spray where the angle and penetration is not controlled during spray administration. For a nasal spray, drug particles are not likely to reach the rhinopharynx region, whereas a significant deposition rate is consistently observed when using the RetroNose device. Deposition images show a more distal and homogeneous deposition in the VCU nasal model when using the RetroNose device compared with a nasal spray (Figure 4).
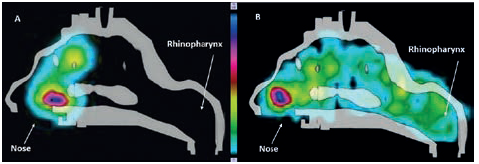
Figure 4: Radioactive deposition of fluticasone in the VCU model with Flixonase® nasal spray (A), and Flixotide® RetroNose prototype device (B).
DISCUSSION
The deposition differences between a nasal spray and the RetroNose device can be explained by the particle size and the route of administration. The particle size generated by the RetroNose device is smaller (12 μm) than the nasal spray (47 μm) and therefore the kinetics of the particles in the nasal cavities is different, resulting in different deposition between the two. Administration through the mouth also induces a difference in air humidity compared with nasal administration, which can increase the size of the particles.
Particle intake from the oropharynx instead of the nose can also explain the difference in terms of penetration and deposition in the different regions of the nasal cavities.
Depositions in the mouth and oropharynx using the RetroNose device can be explained by the particle velocity generated by the pMDI. Similar deposition has been reported when using commercialised pMDIs for inhalation.
The homogenous deposition (Figure 3) obtained with the RetroNose prototype device demonstrates its ability to administer the corticosteroid directly into the different anatomical regions of interest, including the sinuses. This deposition pattern is very different to that of the nasal spray, where the drug is deposited in the first centimetres of the nose. Similar homogenous depositions have been reported using a nebuliser, and have demonstrated increased drug retention in the nasal cavities and higher clinical efficacy than a nasal spray.1 In this study, the RetroNose device used a pMDI as an aerosol generator and can be defined as a portable, multidose device for nasal delivery without lung deposition.
CONCLUSION
This study has shown promising results with regard to a new method of nasal drug administration for producing a more homogenous deposition distribution than a standard nasal spray. The RetroNose device demonstrated potential to be of interest for local, vaccine and systemic nasal drug delivery.
ACKNOWLEDGEMENT
The authors would like to thank Dr P Worth Longest of Virgina Commonwealth University for providing the upper airways model.
REFERENCES
- Reychler G et al “Effect of three drug delivery modalities on olfactory function in chronic sinusitis”. Laryngoscope, 2015, Vol 125(3), pp 549–555.
- Laube BL, “Devices for aerosol delivery to treat sinusitis”. J Aerosol Med, 2007, Vol 20 Suppl 1, pp S5–S17.
- Sutton S, “Navigating the Nose”. The Medicine Makers, 2017.
- Vecellio L, Le Pennec D, Regard A, “Nasal drug delivery via the oral route using a pMDI”. ONdrugDelivery Magazine, Issue 92 (Nov 2018), pp 48–50.
- Kobayashi Y et al, “A novel therapeutic use of HFA-BDP metered dose inhaler for asthmatic patients with rhinosinusitis: Case series”. Int J Clin Pharmacol Ther, 2014, Vol 10, pp 914–919.
- Kobayashi Y et al, “HFA-BDP metered-dose inhaler exhaled through the nose improves eosinophilic chronic rhinosinusitis with bronchial asthma: a blinded, placebo-controlled study”. Front Immunol, 2018, Vol 9, p 2192.
- Valentine R et al, “A prospective controlled trial of pulsed nasal nebulizer in maximally dissected cadavers”. Am J Rhinol, 2008, Vol 22(4), pp 390–394.
- Durand M et al, “Plastinated nasal model: a new concept of anatomically realistic cast”. Rhinology, 2011, Vol 49, pp 30–36.
- Le Guellec S et al, “Validation of anatomical models to study aerosol deposition in human nasal cavities”. Pharm Res, 2014, Vol 31(1), pp 228–237.
- Longest PW, Tian G, Hindle M, “Improving the lung delivery of nasally administered aerosols during noninvasive ventilation— an application of enhanced condensational growth (ECG)”. J Aerosol Med Pulm Drug Deliv, 2011, Vol 24(2), pp 103–118.
- Chand R et al, “Radiolabeling validation of fluticasone propionate/salmeterol xinafoate – HFA pressurized metered dose inhaler”. Resp Drug Delivery, 2012, pp 573–575.

