To Issue 164
Citation: Lee C, Stout M, “Designing a More Versatile and Modern Wearable Device”. ONdrugDelivery, Issue 164 (Sep 2024), pp 24–27.
Chaoyoung Lee and Mike Stout introduce the Horizon wearable micro-pump platform, a cutting-edge advancement in the drug delivery device market that marks a significant contribution to wearable drug-device design.
TxSphere’s extensive experience with infusion pumps and combination drug delivery devices has provided valuable insights into the shift towards subcutaneous (SC) self-administration of pharmaceuticals. These insights prompted the company to explore whether this growing trend has resulted in any significant unmet needs.
THE SELF-ADMINISTRATION TREND
SC delivery is preferable to intravenous methods for several reasons. First and foremost, it is considerably less invasive. It can also significantly improve the patient’s quality of life by allowing them to administer their own medication without the need for frequent clinic visits. This not only improves patient autonomy but also reduces healthcare costs.1
Studies into the self-administration trend have highlighted several unmet needs. Key areas that have been identified for improvement are usability and a need to deliver larger doses, as not all biologic drugs can be formulated in small doses.1 In addition, wearables and autoinjectors are predominantly designed as “injectors”, which deliver doses quickly. At some point, larger volumes become impractical for this approach.
These unmet needs prompted TxSphere to investigate if a miniature version of its electromechanical pumping technology could offer a solution. The company envisioned a versatile wearable device capable of both quick injections and prolonged infusions providing flexibility without compromising patient comfort. Such a device can handle a broader spectrum of biological and pharmaceutical agents, including complex regimens requiring high doses and viscosities. The resulting device should be both small and lightweight, suitable for extended wear, and beneficial for both small and large volumes.
TxSphere’s research also identified several key considerations for improving wearable devices:
- Providing the best possible patient experience
- Advancing safe and effective self-administration
- Accommodating a diverse range of drug administration requirements
- Reducing the cost per dose.
“TxSphere formed a dedicated development team, which led to the launch of the Horizon project.”
To make this vision a reality, the company formed a dedicated development team, which led to the launch of the Horizon project. However, it became clear that a more focused design environment would benefit the project’s long-term objectives. This vision came to fruition in early 2024, with a spin-off to establish TxSphere LLC, a specialised organisation devoted to advancing drug delivery devices.
HORIZON III – ADDRESSING MARKET NEEDS
Design Background
The Horizon platform consists of three distinct versions, Horizon I, II and III. While the design elements were altered with each version, the core product design requirements remained the same:
- Minimal impact on daily life
- Ease of use
- Low cost per dose.
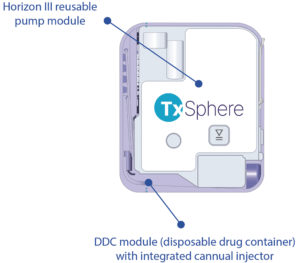
Figure 1: The Horizon III pump and DDC with integrated cannula injector.
All Horizon wearables include three primary components: pump, prefillable drug reservoir and cannula injector. The difference between each version is whether these components are combined or supplied as separate modules.Horizon I combines the three components into a single, compact, pod-like unit. Horizon II introduces three separate modules for each component. Horizon III is the latest and most advanced version – designed with two separate modules, the reusable pump and the disposable drug container (DDC) with integrated cannula injector (Figure 1). This configuration provides a balanced approach to meet the market demands for patient experience, cost efficiency, flexible drug administration and sustainability. The adaptable design also satisfies a wide range of clinician and pharmaceutical manufacturer needs.
Patient Experience
Horizon III is designed for simplicity and ease of use. Its user interface consists of a single start/stop button and requires only three simple steps for operation (Figure 2):
- Place the DDC onto the pump
- Attach the DDC/pump assembly to the skin
- Press the start button.
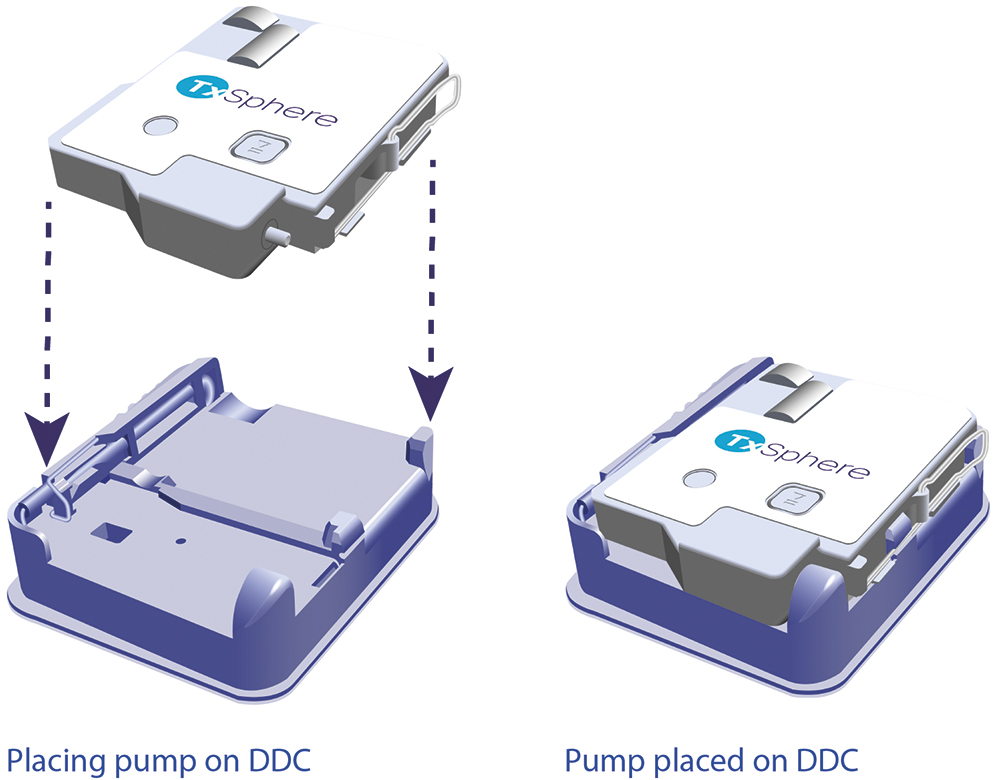
Figure 2: Placing the Horizon III pump onto the DDC.
Instead of using a needle, Horizon devices use an autoinjector mechanism to insert a soft cannula, which enhances comfort, especially during extended infusions. The hidden cannula injector activates automatically, requiring no user intervention. For many, this feature can help alleviate the apprehension associated with self-injection.
Horizon III is prefillable, which reduces the complexity and risk from self-filling the drug reservoir. However, for drugs that cannot be prefilled, Horizon wearables can be manually filled at the point of care or can automatically transfer medication from a vial.

Figure 3: Horizon III – compact design for more comfortable wear.
The custom internal reservoir was a key factor to reduce the size and weight of each Horizon device. This was because there were no restrictions for size, shape, weight and volume of injectable drug cartridges. Even with its standard 10 mL reservoir, Horizon III remains more compact and lightweight than many devices with lower capacities. This small form factor makes the device more comfortable, less noticeable and easier to conceal (Figure 3).
To power the device throughout its lifecycle, a simple wireless charging feature is incorporated into Horizon III. It is also compatible with common wireless mobile phone chargers.
Self-Administration
Ensuring proper drug administration and compliance are among the top clinical concerns with self-administration. To address these concerns, Horizon III is equipped with sensors that verify the completion of all operational steps: placement of the DDC on the pump, removal of the adhesive paper and attachment to the skin.
To ensure that an inadvertent press of the start button does not initiate the infusion, the button is not active until all these steps have been confirmed by the sensors. Additionally, infusion will not begin until the cannula injection has been confirmed. To improve sustainability, these sensors are all located within the reusable pump body for use throughout the lifecycle.
Monitoring for compliance can also help ease concerns. The Horizon platform provides connectivity that can be used for compliance monitoring as well as many other related uses.
Manufacturing
The modular design of the Horizon III enhances manufacturing efficiency. The separate pump and DDC modules each have distinct requirements for environment considerations, regulations, processes, quality control and packaging. Separate modules permit the use of individualised manufacturing lines that can better address the specific requirements of each module. Additionally, the electromechanical pump components can be eliminated from the sterilisation process.
All Horizon devices are designed to be prefilled with medication. The custom internal reservoir comprises a co-polymer film designed specifically for sterile drug product storage. TxSphere’s patented fill port makes the prefilling process simpler and more efficient.
Drug Administration Requirements
Due to the limitations of the SC tissue’s capacity to absorb medication, the volume of medication administered subcutaneously is typically between 1 and 2 mL. But complex molecules, such as monoclonal antibodies, may require larger doses. Strategies to deliver higher volumes include divided doses, smaller volumes or more frequent dose administration.1 Other strategies include slower delivery speeds for longer delivery times and drug reformulation.
Although hand-held autoinjectors are now able to deliver up to 5.5 mL, they are limited by how long a user can be expected to hold the device against their skin to complete a delivery.2,3 Slower injection speeds can increase the amount of fluid that can be delivered, help reduce the pain caused by quick injections and increase drug dispersion and absorption.4,5 For example, SC immunoglobulins are highly viscous biological products with single injection site doses as high as 50–60 mL. To accommodate these volumes, they are delivered at low speeds of 1 mL/minute or less.6,7
Horizon III’s robust pumping mechanism can deliver highly viscous solutions at various delivery speeds and infusion times. The flexible design of the internal drug reservoir makes it possible to accommodate larger dose volumes. With the standard footprint, Horizon can hold up to 20 mL and can also accommodate higher volumes with an expanded footprint.
“With the ability to deliver complex regimens, Horizon III can provide a cost-effective alternative to drug reformulation.”
With the ability to deliver complex regimens, Horizon III can provide a cost-effective alternative to drug reformulation. The use of innovative SC delivery devices can even facilitate larger-volume SC injections without the need for permeation-enhancing enzymes.8
Sustainability and Environmental Impact
Sustainability can be enhanced through both manufacturing processes and considerations for device disposal.9 According to the WHO, treatment and disposal of healthcare waste may pose health risks indirectly through the release of pathogens and toxic pollutants into the environment. They define infectious waste as anything contaminated with blood and other bodily fluids.10 This concern is elevated when additional items are discarded with the infectious waste.
When injection needles and cannulas are combined with the delivery component, the entire device must be disposed of as hazardous waste after each use, which is neither environmentally sustainable nor cost effective. Because of the reusable pump module, the Horizon III pump can be reused and recycled. This means that the DDC is the only component that needs to be be disposed of as hazardous waste after each dose.
Cost Reduction
The Horizon III micro-pump module is the most expensive component. Because it is designed for extensive reuse, the cost per dose is spread out over many injections. As a result, the cost per dose is driven primarily by the less expensive disposable DDC.
THE HORIZON PLATFORM
Not only is each Horizon version different in how the three primary components are combined, but they are also different in how they address various market requirements. Horizon I is optimised to provide the best patient experience. Horizon II introduced improvements to manufacturability, sterilisation, sustainability and cost per dose. Horizon III is a hybrid that incorporates the best features of Horizon I and II. The Horizon I and II designs and features are compared below.
Horizon I
Horizon I combines the three components (pump, drug reservoir and cannula injector) into a single, compact, pod-like unit. It is remarkably small and simple, and can be supplied prefilled with medication. Offering all three components in a ready-to-use form, it provides the best patient experience (Figure 4).

Figure 4: The Horizon I all-in-one wearable injector.
However, the all-in-one design presents several challenges in terms of manufacturability, sterilisation, sustainability and cost per dose. The sterilisation process is complicated by the inclusion of electronic and mechanical components packed into a very small space. Additionally, the all-in-one design means that the entire device must be discarded as hazardous waste after each dose, which affects cost and environmental impact.
Horizon II
To provide improvements to manufacturability, sterilisation, sustainability and cost per dose, Horizon II is based on a modular concept. The pump, drug reservoir and cannula injector are all supplied as separate modules (Figure 5).

Figure 5: The Horizon II reusable pump attached to the DDC module.
These separate modules allow the electromechanical pump portion to be reused, while the sterile components can be handled and disposed of more effectively. This reduces the environmental impact and cost per dose. At the same time, only the DDC module requires sterilisation, which significantly simplifies the process and provides additional cost reductions.
The third module is the cannula injector. Several cannula injectors are readily available primarily due to their use with insulin pumps. Therefore, instead of designing a proprietary version, TxSphere simply added a luer connector to the DDC so that it can connect to the tubing of these cannula injectors.
SUMMARY
TxSphere has introduced the Horizon III wearable micro-pump, a significant advancement in wearable drug delivery technology. It simplifies the self-administration of SC medication with a patient friendly, attach-and-go infusion system.
Horizon III offers a unique platform for differentiating combination pharmaceuticals by enhancing the patient experience, providing flexibility in drug delivery and reducing costs. This model features a user-friendly design with simple operation steps and a soft cannula injector for added comfort. Its prefill capability, flexible platform and small, lightweight design make it an ideal device to deliver a wide range of drug formulations, including large-volume and viscous pharmaceuticals.
REFERENCES
- Dychter, SS et al, “SC Drug Delivery, A Route to Increased Safety, Patient Satisfaction, and Reduced Costs”. J Infus Nurs, 2012, Vol 35(3), pp 154–160.
- Parker M, Chellappan K, Cottenden D, “Directions for Wearable On-Body Injector Systems and Beyond”. ONdrugDelivery, Issue 151 (Sep 2023), pp 12–15.
- Boyd M, “Would You Like to Go Large? Key Considerations for Large-Volume Injectors”. ONdrugDelivery, Issue 151 (Sep 2023), pp 17–21.
- Pepin XJH, Grant I, Wood JM, “SubQ-Sim: A SC Physiologically Based Biopharmaceutics Model. Part 1: The Injection and System Parameters”. Pharm Res, 2023, Vol 40, pp 2195–2214.
- Kim H, Park H, Lee SJ, “Effective method for drug injection into SC tissue”. Sci Rep. 2017, Vol 7(1), p 9613.
- “Cuvitru”. Prescribing information, Takeda Pharmaceuticals, March 2023.
- “Hizentra”. Prescribing information, CSL Behring, April 2023.
- Adler M et al, “Transitioning from Vial to SC Injection Devices for Biological Drug Products”. ONdrugDelivery, Issue 160 (May 2024), pp 8–14.
- Osorio S, Mou S, Dean C, “Sustainable by Design: Developing Patient- and Planet-Centric Medical Devices”. ONdrugDelivery, Issue 159 (Apr/May 2024), pp 52–57.
- “Health-care waste”. WHO, Feb 2018.

