To Issue 151
Citation: Boyd M, “Would You Like to Go Large? Key Considerations for Large-Volume Injectors.” ONdrugDelivery, Issue 151 (Sep 2023), pp 17–21.
Malcolm Boyd discusses the factors that need to be considered early in the development of large-volume injectors, which will have a major influence on the performance, usability and cost of a device.
“The format of the primary container is one of the decisions that will have the greatest impact on the product’s development direction and overall format.”
There is a growing need for injection devices that can deliver higher dose volumes than have traditionally been self-administered in the past. It seems that yesterday’s large dose is rapidly becoming today’s normal dose for some indications.
Handheld autoinjectors and variable dose pens used to typically max out at 0.8–1.0 mL for a single dose. This was then raised to 2.25 mL in devices from leading companies around 2019 – and even to 5.5 mL in the latest variants from 2022. As single-dose volume has increased in handheld devices, much has been discussed about how long a user can comfortably hold a pen-like device on their skin for reliable dose delivery. This holding time consideration has come hand in hand with a growth in the understanding of the body’s tolerability for a large-volume dose delivered in a single injection.
The move to increasingly large injection volumes has been further influenced by both micro and macro trends from within the medical sector, as well as wider socio-economic trends. For example, as a result of the covid-19 pandemic, there was a near overnight shift to online and virtual engagement across the world in all industries. This has ultimately led to an accelerated need for, and acceptance of, healthcare delivery outside the clinic.
When combined with a forecast growth in the global population of more than one billion people in the next 20 years and the fact that it is estimated that approximately a third of all adults already suffer from multiple chronic conditions, the prevalence and cost of managing chronic disease is only going to grow. Increasing the availability and quality of home healthcare offers a way forward in addressing the associated challenges, as well as having the potential to reduce healthcare costs. Furthermore, “disconnecting” patients from intravenous poles by offering at-home injection solutions, where possible, has the potential to increase quality of life – not just for the patient but for their family and friends too.
Such market-influencing factors, combined with the growing injectable drug market and the continued need to reduce needle injury risk and save costs overall, are driving exciting opportunities in drug delivery, particularly for devices capable of delivering large-volume doses in patient-centric ways. The combination of these considerations has led to a growing interest in wearable autoinjectors capable of delivering a fixed dose of 2, 5, 10 and 25+ mL in a matter of minutes, without the need for the patient to hold them for an extended period of time.
When it comes to actually putting pencil to paper, however, and beginning to consider what sort of embodiment may be appropriate to address the market needs for large-volume injectors, there are a number of important aspects that need to be considered early on that will have a major influence on the direction of development and, ultimately, the performance, usability and cost of the device.
“The cost per treatment is directly influenced by device cost. Device cost is, in turn, often linked to functionality and sophistication.”
PRIMARY CONTAINER AND FLUID PATH
At the heart of the device, the primary container must hold the drug in a sterile and stable environment over the lifetime of the filled device. The format of the primary container is one of the decisions that will have the greatest impact on the product’s development direction and overall format. There are generally three main routes for integrated primary containers, with selection influenced by a number of factors, including long-term storage implications, drug stability, device form factor, manufacturing implications, filling strategy and compatibility with the drive system:
- Rigid glass container: glass containers offer excellent chemical durability but are fragile during transport and handling and weigh more than their plastic counterparts. They are also limited in their form and closure mechanism.
- Rigid plastic container: materials such as cyclo-olefin copolymer (COC) and cyclo-olefin polymer (COP) may offer more flexible form options with better tolerances, while maintaining good stability. However, care must be taken to ensure that gas permeation is minimal and any leaching does not affect drug performance.
- Flexible bag: multilayer constructions can potentially provide robust yet flexible pouches for drug storage. Additives or foils can be used to improve gas barrier properties, although long-term storage capabilities may be dependent on the specifics of individual drugs. Flexible bags can also be an attractive option for “fill at time of use” scenarios, where the drug is dispensed into the container from a vial or prefilled syringe by the user and is therefore only in transient contact.
“Consideration of the possible impact of the drive system, primary container and fluid path choices on the drug integrity should not be overlooked.”
Directly linked to the primary container and filling strategy is the device’s fluid path. If a device contains a prefilled primary container, it is not generally appropriate to sterilise the device with a drug on board and definitely not acceptable to introduce a non-sterile fluid path to a primary container or patient. As a result, a fluid transfer strategy needs to be conceived to enable movement of the drug from the container to the outlet/patient without compromising sterility. This will require consideration of factors such as container closure integrity, needle sterility, needle gauge, the point at which fluid connection is made and the point at which drug is introduced to the system, as well as the implications for assembly, packaging, storage and transportation of device sub-assemblies and the final product. This is a critical interface and one that needs careful technical and usability consideration to balance potential additional steps and risks against other benefits.
DRIVE SYSTEM
The choice of drive system is very closely linked to the choice of primary container. The selection and integration of the drive system has to be considered in the context of whether the device is disposable or reusable, whether it is body-worn or not and the fluid path strategy, as well as configurability if it is intended to be a platform device – i.e. how easy it is to modify the drive system to accommodate, for example, different dose sizes, flow rates and viscosities. Each combination of decisions will impact device architecture and the user workflow, so must be considered together for optimal design efficiency and output.
Specifically thinking about the choice of drive system, at a high level there are push systems and pull systems. Push systems are the more traditional design, driving a piston forward to expel fluid, whereas pull systems draw the fluid from a reservoir. In pull systems there is the added consideration of whether or not the mechanism is in contact with the fluid – each option has its own advantages and disadvantages, depending on other architectural decisions.
Push systems are generally better suited to rigid containers, whereas a pull system could feasibly draw from any container, potentially offering the most flexibility – and therefore smallest package size. In both instances, consideration of the possible impact of the drive system, primary container and fluid path choices on the drug integrity should not be overlooked.
DISPOSABLE OR REUSABLE
The cost per treatment is directly influenced by device cost. Device cost is, in turn, often linked to functionality and sophistication. It therefore follows that a simple, easy-to-use device, minimising cost and environmental impact, is a good route forward. However, in some cases it may be appropriate, or necessary, to introduce more advanced functionality – for example, to support user feedback, adherence, monitoring, connectivity, platform flexibility, etc. Such a sophisticated device may have a high cost of goods, making a disposable version both financially and potentially environmentally unattractive. A reusable embodiment may offer a significantly cheaper and more sustainable route if reused a sufficient number of times.
Where sophisticated features are needed, reusable and partially reusable devices offer significant potential for cost-effective and environmentally sustainable solutions through the reuse of high-value parts and sub-assemblies, such as motors, display screens and batteries. This approach potentially makes features such as enhanced feedback and connectivity more viable by “amortising” the cost of the device over an extended life. However, these advantages nearly always come with additional user task steps that, in turn, can create the potential for additional use errors.
With this in mind, and notwithstanding the above points, it is crucial to understand and consider the wider treatment ecosystem when making decisions on disposable versus reusable strategies. For example, a sophisticated disposable device may ultimately be more environmentally and financially appropriate in some circumstances – for example, where a reusable approach is considered too challenging for a particular user group, potentially leading to poor adherence and increased hospital visits and stays, all of which add cost and environmental burden in the bigger picture. If developing a reusable system, a particularly important consideration is the injection needle and associated fluid path. This is normally a single-use, disposable item and therefore requires parallel consideration of device interlocks and the logistics of manufacturing and distributing replacement fluid paths.
Commensurate with the projected life of the device, in any design, the mechanisms to permit appropriate reuse and/or prevent inappropriate reuse need consideration. Furthermore, for reusable devices, it is also key to consider capability to recharge power, where needed, and replace disposable elements safely and easily when required.
Whether disposable or reusable, the development approach for each strategy requires a considered device architecture and user interaction experience and workflow, as well as an understanding of disposal regulations in the markets being targeted.
Different scenarios may lead to different conclusions but – in combination with the other points outlined – whether a device is to be disposable or reusable will have a strong influence on its detailed design and should therefore be considered early on, in particular to weigh up added user benefits from a more sophisticated reusable system against the additional user task steps and associated risks it may introduce (Figure 1).
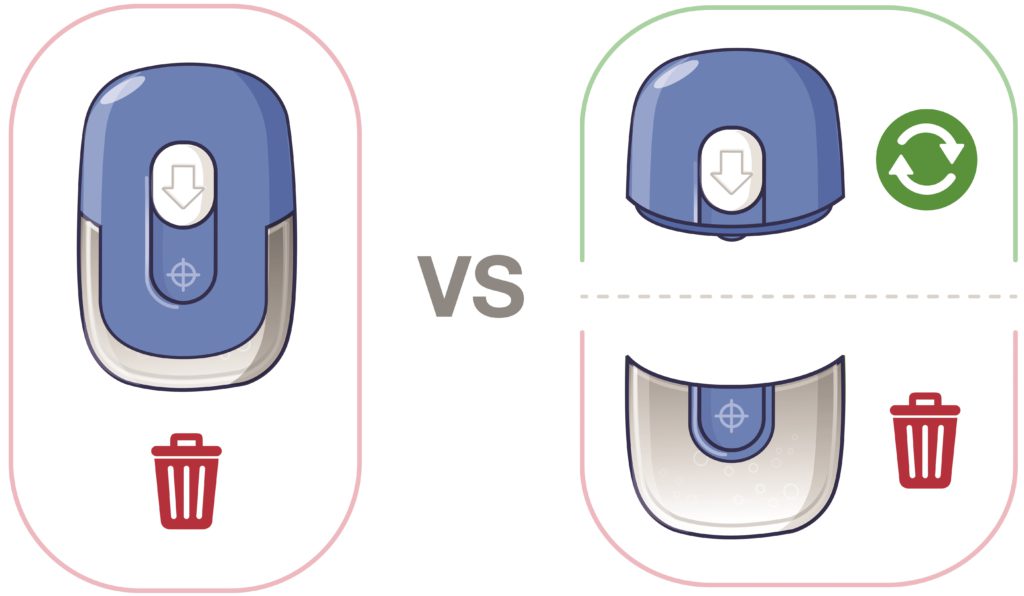
Figure 1. Disposable, reusable or partially reusable?
BODY-WORN OR NOT
Whether a disposable or reusable route is taken and whether development is a “platform” or a targeted embodiment will, in combination with dose volume, influence whether a device is better designed to be specifically body-worn or not. Patient satisfaction is also likely to be influenced by the comfort of wearing/interacting with the device, along with other factors such as ease of use, frequency of use, injection time, the injection process and size.
Fundamentally, the level of “inclusiveness” of the device should be considered. This needs to be assessed at sensory, cognitive and physical levels. A device should be sized to fit the target population comfortably, all interactions should lie within the target population’s physical capabilities (for example, the ability to grip, control and actuate) and it should be possible to easily sense and understand all feedback.
“This is a young and still emerging and evolving device technology space in which no single format has yet been firmly established
as the benchmark.”
Considering these points, for scenarios at the lower end of the large-dose volume range, the optimal solution is most likely to be an integrated/“co-located” body-worn solution. In this scenario, a single enclosure that is directly attached to the skin would contain the drug, needle path and drive mechanism. However, as the dose volume increases, there is likely to be a threshold where a non-body-worn/ distributed system becomes optimal or provides the most flexible platform. This may mean designing part of the system to sit on a table, attach to a belt clip or be housed in a shoulder bag. The exact tipping point will be specific to the embodiment and a factor of the size of the device, the size of the footprint, the total mass, the dose volume and the centre of gravity.
With these and other points in mind, the following core aspects should be considered as part of the decision:
- Handling: as a device becomes larger, it will inevitably become more difficult to handle, particularly when trying to position in the correct place on the body. This difficulty is likely to be further magnified where users suffer from dexterity challenges, such as rheumatoid arthritis or multiple sclerosis. Industry accepted human factors data suggests that dimensions greater than 85 mm are expected to be a problem for some people to grip in one hand. Therefore, assuming one-handed handling, at least one dimension – used to grip when fitting – should be below ~85 mm and ideally lower than 80 mm for a global market.
- Footprint: the footprint of the device may limit the options for positioning Figure 1. Disposable, reusable or partially reusable? and orientation on the body, as well as impact which injection sites are available. Smaller footprints offer greater flexibility, providing more scope for the injection sites to be varied, provided that the smaller footprint is not at the cost of stability, vulnerability to knocks or device removal forces due to a need for stronger adhesive. Various combinations and reflections on these may necessitate moving from body-worn to non-body-worn.
- User steps: a co-located body-worn disposable system will potentially have the least end-user tasks associated with delivering the medication, whereas a non-body-worn distributed reusable system will likely require further steps in managing the various system elements (e.g. the needle port/infusion set, the drive unit and the primary container/filling strategy).
- Fluid path: a body-worn device, even if using a separately connected needle port, is likely to have a shorter fluid path and therefore lower risk of accidental occlusion. Similarly, a short fluid path requires less system priming and therefore results in less dead volume of drug.
- Portability: some indications may not need to consider the ability for the user to move around during drug delivery, but for others this may be a key requirement or an opportunity for differentiation. This affects both body-worn and not, whereby if body-worn, the adhered device needs to be resilient to movement and knocks, and where non-body-worn needs to be easy and discrete to carry around (Figure 2).
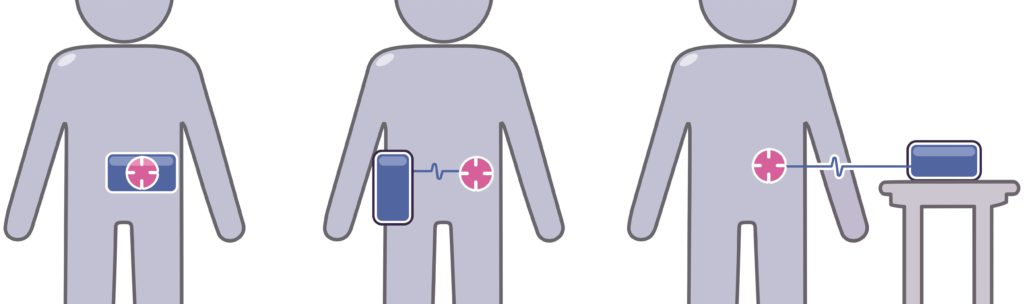
Figure 2. Co-located body-worn, distributed body-worn and distributed non-body-worn options.
THE “PATCHWORK” MARKET
The past few years have seen rapid growth of the biologics and biosimilars markets. This growth has also seen development of very specific molecules that are tailored to treat specific diseases, whether they be specific neurological disorders, autoimmune diseases, oncology or other therapeutic areas. With increasingly tailored treatments and regimens, the specific needs for delivery of each drug and each patient can vary subtly or significantly between relatively small markets. While more markets and the existence of more opportunities is exciting, it comes with the challenge of managing return on investment when developing a device for use across multiple “niche” markets.
It is key, therefore, to explore and align on an appropriate development and product strategy based on internal and external commercial and market information early on.
It may be that the appropriate route is to develop a sophisticated modular platform that can be adapted efficiently to address a number of market opportunities (e.g. flexible for various volumes, body-worn or portable with infusion set, etc.) or, conversely, to develop a very specific embodiment for a focused target. With each approach having particular pros and cons, considering and defining a target market or markets is a key factor in formulating a successful development strategy (Figure 3).
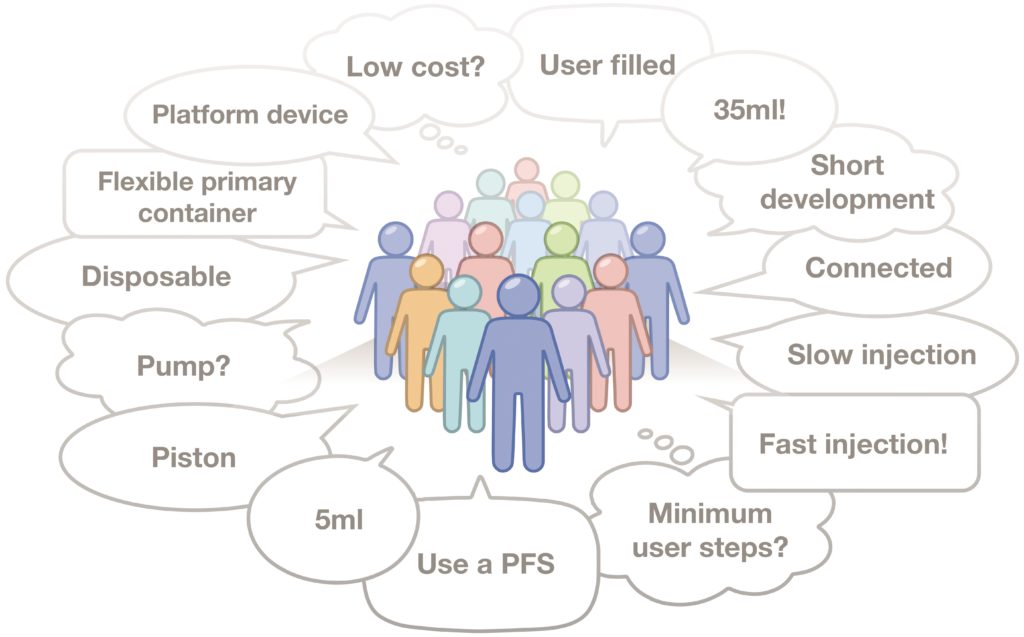
Figure 3. Many voices from the market need to be considered when forming a development strategy.
SUMMARY
A range of socio-economic and industry influences are increasing the number of opportunities for larger volume injection devices but the market “pull” isn’t entirely straightforward at present. However, this is a young and still emerging and evolving device technology space in which no single format has yet been firmly established as the benchmark (unlike pens and autoinjectors, which are much more mature). As a result, there is arguably still significant potential for interesting new concepts and strategies to emerge. The winners in this space will be the products that find the best balance of the key factors, such as those discussed in this article, for their target market and user populations, and use informed, evidence-based decision making as a foundation for efficient and effective device development.

