To Issue 154
Citation: Belli B, “Developing an Inhalable Formulation of the First Generative AI Drug”. ONdrugDelivery, Issue 154 (Nov 2023), pp 6–8.
Brita Belli introduces Insilico Medicine’s inhalable formulation of its AI-designed therapy for the treatment of idiopathic pulmonary fibrosis, its progression through clinical trials and the company’s approach to generative AI in the pharmaceutical space more broadly,
On the heels of announcing Phase II trials for the first generative artificial intelligence (AI) drug, targeted for idiopathic pulmonary fibrosis (IPF), Insilico Medicine announced another breakthrough – an inhalable solution for its anti-fibrotic small molecule inhibitor, designed to give patients another treatment option. The new formulation has been announced as a preclinical candidate, and the company is proceeding with an IND filing. Insilico is now the first AI drug discovery company to venture into nebulised formulations.
“The only two FDA-approved therapies for IPF are the oral treatments pirfenidone and nintedanib, which may provide some relief or slow the progression of the disease but do not reverse the damage or stop it entirely.”
“At Insilico, innovation never stops, and that means multidimensional validation of our AI platform capabilities, for various disease areas, indications and formulations,” said Dr Feng Ren, Co-Chief Executive Officer and Chief Scientific Officer of Insilico Medicine. “We hope to advance the inhalation formulation of ISM001-055 to clinic as soon as possible to address unmet clinical needs.”
ADDRESSING THE LIMITATIONS OF CURRENT IPF TREATMENTS
IPF is a chronic progressive scarring disease of the lungs, in which a combination of excessive extracellular matrix deposition and alveolar tissue results in a build-up of scar tissue that leads to an irreversible decline in lung function, followed by lung failure and death. This rare disease affects approximately five million people worldwide, with a median survival time of just three years.
The only two US FDA-approved therapies for IPF are the oral treatments pirfenidone and nintedanib, which may provide some relief or slow the progression of the disease but do not reverse the damage or stop it entirely. These medications also need to be taken in high doses and can cause unpleasant side effects. For example, the required daily dose of pirfenidone is nine pills, and studies show that patients on this regimen often experience adverse side effects, including nausea, abdominal pain and photosensitive rash. Meanwhile, more than 60% of patients in a nintedanib treatment group experienced diarrhoea.1
In addition to exploring new mechanisms for treating IPF, companies like Insilico are also looking at new formulations – specifically inhalable treatments – that could deliver therapies directly to the lungs to treat the disease with minimal off-target effects.
Initial studies have indicated that the inhaled formulation of Insilico’s potentially first-in-class small molecule inhibitor, INS018_055, has the potential to address some of the limitations of current therapies (Figure 1). “Through inhalation, we can achieve high efficacy, high bioavailability and limit side effects, especially gastrointestinal-related side effects,” explained Dr Ren.
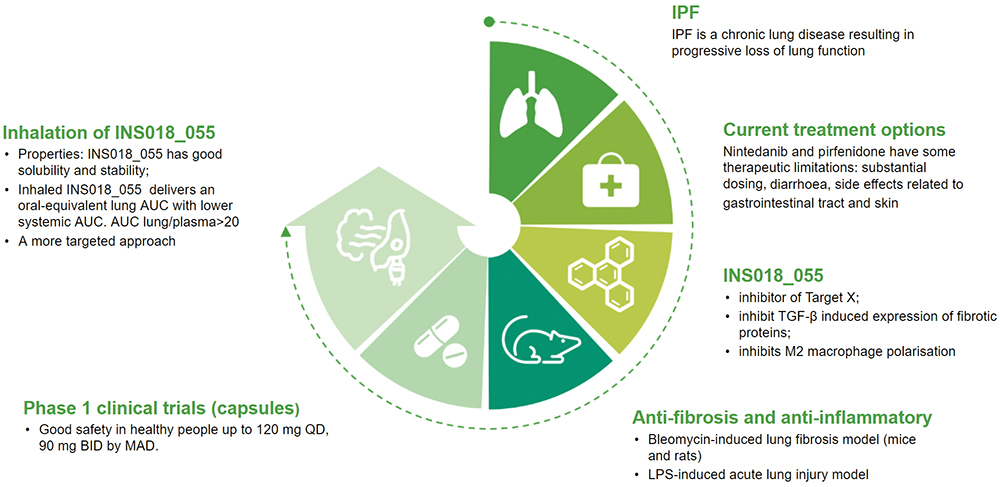
Figure 1: Infographic detailing INS018_055.
“With the potential benefits offered by inhalable formulations, a number of companies are exploring the space for their own IPF treatments.”
WHY AN INHALABLE FORMULATION?
While Insilico is continuing to advance its lead oral drug INS018_055, which has demonstrated promising results in multiple preclinical studies and was shown to have potential relevance in a broad range of fibrotic indications, the company hopes to expand the potential treatment options with its inhalation formulation. Inhalation of INS018_055 could provide a more targeted means to deliver the drug to the lung with an oral-equivalent area under the curve (AUC) but lower systemic AUC, as well as rapid onset of action, high bioavailability and lower required dose.
With the potential benefits offered by inhalable formulations, a number of companies are exploring the space for their own IPF treatments (Figure 2). While Insilico’s is the first AI-designed inhalable IPF drug formulation in development, it joins a number of others in the field. For example, there is an inhalable version of pirfenidone by Avalyn Pharma (WA, US) that has shown favourable tolerability and is in Phase Ib studies.2 Meanwhile, GSK is advancing the IPF drug GSK3008348, which is administered by a nebulised spray and is also presently in Phase I trials to determine safety and efficacy in healthy volunteers.3
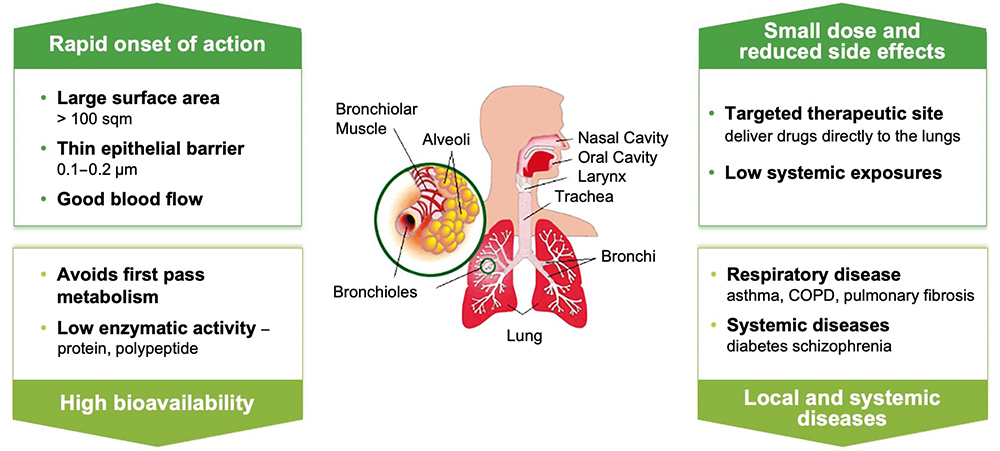
Figure 2: Infographic detailing the advantages of drug delivery to the lung for respiratory conditions.
“The AI transformation of the pharmaceutical industry is well underway. Insilico is one of the lead players in this effort.”
THE PATH TO INSILICO’S INHALABLE IPF DRUG
Insilico’s inhalable IPF drug formulation began its journey in 2021, when the company announced it had selected a preclinical candidate. The company used its AI target identification engine, PandaOmics, to identify a novel pan-fibrotic target, dubbed “Target X”. Insilico’s generative AI small-molecule engine, Chemistry42, was then applied to Target X to design an entirely new molecule from scratch that could be explored as a new IPF treatment option. The entire process was accomplished in record time – under 30 months from target discovery to Phase I trials.
In January 2023, the Phase I trials had produced positive topline results and, in February 2023, Insilico’s oral IPF drug received ODD from the FDA. This lead drug has now begun Phase II trials with patients, the first AI-discovered and AI-designed drug to reach this milestone.
“Initiating Phase II trials with this novel inhibitor for IPF represents a major milestone for deep generative reinforcement learning in drug discovery,” said Insilico founder and Co-Chief Executive Officer Alex Zhavoronkov. “We will explore the efficacy for patients of AI-discovered and designed treatments in clinical trials, which is a true validation of our generative AI platform. We are eager to continue to advance this potentially first-in-class therapy forwards to help patients in need and show the value of generative AI in drug discovery and development.”
THE AI TRANSFORMATION OF PHARMA
The AI transformation of the pharmaceutical industry is well underway. Insilico is one of the lead players in this effort, having developed an end-to-end pharma AI platform that has been used to develop a pipeline of over 30 drugs in development across cancer, immunity, fibrosis and central nervous system diseases. Four of these AI-designed drugs are currently in clinical stages.
Pharma companies license both Insilico’s AI-designed and developed therapeutic assets and its software to advance their internal drug development programmes. The company recently signed a strategic agreement with Exelixis (CA, US), giving the oncology-focused biotech the right to develop and commercialise ISM3091, a potentially best-in-class small-molecule inhibitor of USP1, which has emerged as a synthetic lethal target in BRCA-mutated tumours. The agreement included a US$80 million (£66 million) upfront fee, in addition to milestone payments and royalties on net sales.
Additionally, in November 2022, Insilico announced a multi-target collaboration with Sanofi to use its AI platform to advance drug development candidates for up to six new targets with upfront and target nomination fees of up to $21.5 million and other development and commercial-based milestones that could total up to $1.2 billion, in addition to royalties.
The latest research reveals that bringing a single drug to market now costs over $6.1 billion for a big pharma company.4 In a more nimble biotech setting, driven by the speed and efficiency of AI, faster and less expensive high-quality early drug development is possible. Strategic partnerships between big pharma companies and biotech start-ups can take these AI-designed drugs over the finish line. What began as the first fully generative AI drug to reach Phase II trials, and expanded to become the first inhalable formulation of a generative AI drug, could ultimately come to represent one of the first steps in a major industry shift.
REFERENCES
- Ghumman M et al, “Emerging drug delivery strategies for idiopathic pulmonary fibrosis treatment”. Eur J Pharm Biopharm, 2021, Vol 164, pp 1–12.
- “Avalyn Pharma Presents Data Showing Favorable Tolerability and Potential of AP01 to Preserve Forced Vital Capacity in Adults with Pulmonary Fibrosis”. GlobeNewswire, Sep 2023.
- “First Time in Human (FTIH) Study of GSK3008348 in Healthy Volunteers and Idiopathic Pulmonary Fibrosis Patients”. ClinicalTrials.gov, May 2017.
- Schuhmacher A et al, “Analysis of pharma R&D productivity – a new perspective needed”. Drug Discov Today 2023, Vol 28(10), p 103726.

