To Issue 141
Citation: Ben Jamaa H, “Digital Health: Addressing Modern Healthcare Challenges with Sensors”. ONdrugDelivery, Issue 141 (Dec 2022), pp 16–20.
Haykel Ben Jamaa explores why modern society needs digital health and how it is organised and implemented, with a focus on sensing technologies empowering digital health technologies.
“Dealing with the healthcare consequences of the ageing population requires health, activity and medication monitoring.”
Digital technology is playing an increasingly critical role in modern healthcare. As society faces new healthcare challenges – including a rise in chronic diseases and an ageing population – public health services will increasingly benefit from the growing role of digital technology. The WHO defines digital health as “the field of knowledge and practice associated with the development and use of digital technologies to improve health”.1 The term has far-reaching implications and encapsulates a wide range of digital technologies, from software solutions, which enable better management of patient data and provide easier access to patient support services, to new diagnostic tools and smart drug delivery devices.
ARE MODERN HEALTH CHALLENGES A DRIVER FOR DIGITAL HEALTH?
Globally, healthcare providers are dealing with an increase in the incidence of noncommunicable diseases, such as cardiovascular diseases, cancers, diabetes and chronic respiratory diseases – primarily chronic obstructive pulmonary disease (COPD) and asthma. On the one hand, this is a result of a reduced number of deaths from infectious diseases, such as tuberculosis. Nevertheless, as noted by the WHO, noncommunicable diseases pose devastating health consequences and socioeconomic effects, which make their control a major challenge that needs to be addressed.2
The occurrence of such diseases is increasing in line with the world’s ageing population. As birth rates decline and healthcare improves in many regions, the WHO estimates that the proportion of the world’s population over the age of 60 will almost double by 2050. Dealing with the healthcare consequences of the ageing population requires health, activity and medication monitoring. Hospital stays are costly and increase the risk of infections, so drug delivery in a homecare setting, perhaps with remote monitoring, is expected to reduce healthcare costs and improve outcomes.3
At the same time, environmental conditions are also amplifying the incidence of some of these diseases. The increase of ultrafine particles in the air due to urban traffic, industrial processes, pollution and the increasing frequency of wildfires are worsening environmental conditions for patients with asthma and COPD, as well as being linked to increased rates of diseases such as cancer and even dementia.
Digital health plays a vital role in helping healthcare systems evolve to meet these challenges. Digital health already plays an undeniable role in modern healthcare, from online appointment booking through continuous glucose monitors to digitising patient records. Many of these provisions – such as virtual platforms and video call appointments – were expedited by the response to the covid-19 pandemic, as necessity pushed through years’ worth of reforms in the space of just a few months.4 Following this recent push, the infrastructure of digital health is now being defined and consolidated by governments, healthcare providers and industrial partners.
HOW IS DIGITAL HEALTH ORGANISED AND IMPLEMENTED?
“Digital health” is a broad, all-encompassing term for the application of digital technology to healthcare. Organisation of healthcare services, such as real-time digital patient data management, medical act management and billing tools, is one of the key ways in which digital health is currently implemented. However, digital health means more than just this.
Arising applications of digital health include new types of medical devices, such as smart injection devices, that implement new monitoring and diagnostic functions. These medical devices are used in direct proximity to the patient, often as wearable or hand-held devices, such as insulin pumps or smart inhalers, respectively. Moreover, new consumer wellbeing devices, such as smart watches, sleep monitors and breath analysers, provide the public with additional health-related information.
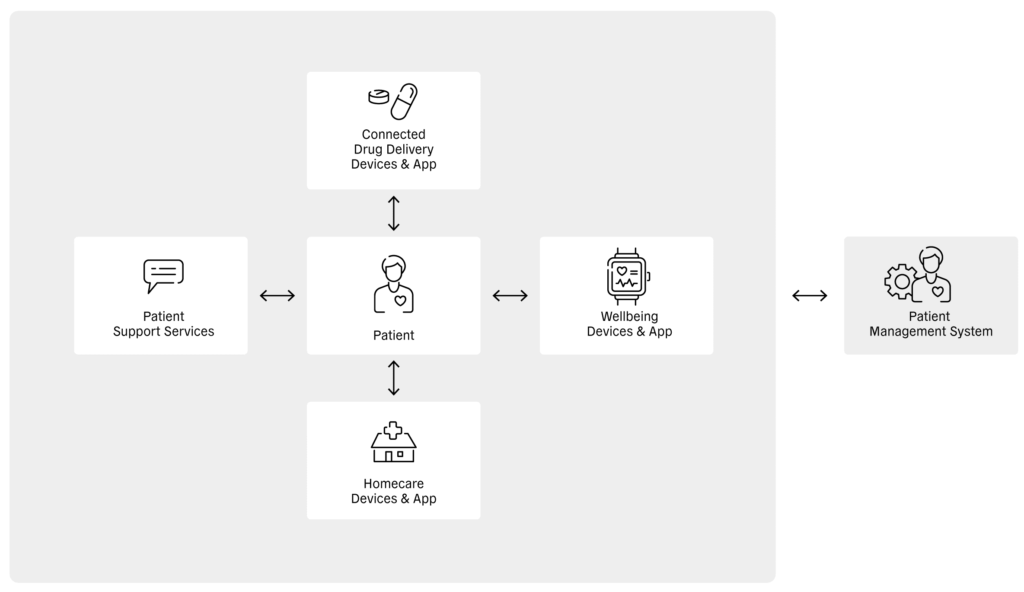
Figure 1: Relationship between the patient, different healthcare apps, patient support services and the patient management system.
At the patient level, digital health enables health-related information to be shared directly between the patient and connected drug delivery devices, wellbeing devices, homecare devices and patient support services (Figure 1). In turn, these devices, services and apps can transfer patient information to service providers, where it can be stored and analysed. Sharing this information with healthcare providers enables diagnoses, treatments and disease-management procedures to be assessed, which can, in turn, be relayed to patient management systems and shared with payors (Figure 2).
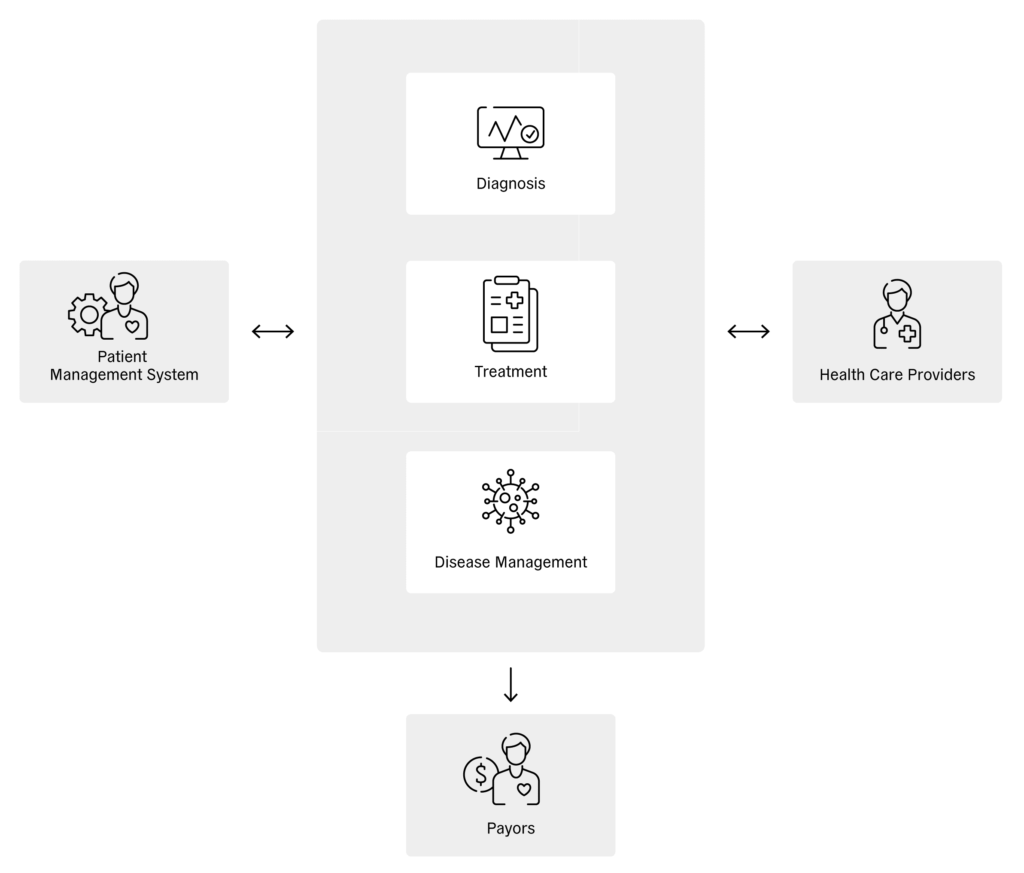
Figure 2: Reciprocal influence between patient management system, therapy and healthcare providers.
“Choosing an optimal sensor solution can be challenging from a
design perspective, as it must fulfil its intended function and seamlessly integrate into the patient’s user experience.”
The increased availability of health-related patient data can play an important role in drug development, enabling the realisation of personalised therapies or drugs on a large and practical scale. Moving further along the healthcare value chain, connected medical devices make it easier for patients and caregivers to monitor healthcare and manage the therapies.
Furthermore, clinical trials and research studies have highlighted the potential of digital health technologies to reduce non-adherence.5 Non-adherence to asthma treatment is a major issue that causes disease exacerbation, hospitalisations and high financial costs. However, patients using the next generation of smart inhalers can monitor the quality of their inhalation, obtain feedback on the drug delivery efficiency and even on the course of the disease. The adherence of these patients could be significantly improved, leading to improved outcomes and lower healthcare costs.
Given the high value for the patient, partnerships are forming between pharma companies and technology companies around the world, resulting in the development of a spectrum of new drug delivery devices.6 For example, GSK recently partnered with Propeller Health to develop a digital inhaler and AstraZeneca has partnered with Adherium to bring an adherence monitor to market to support AstraZeneca’s Turbuhaler (budesonide and formoterol) medication.7,8
The implementation of digital health is also being supported by governments. New legislation, such as the DiGA in Germany and Article 51 in France, is laying the foundation for digital health by supporting new care pathways, such as the prescription of digital health apps by healthcare professionals.9,10 The extended definition of remote patient monitoring in the US with additional Current Procedural Terminology codes since 2022 is also further advancing the use of connected devices.11 These devices enable monitoring of non-physiological information to help assess patient adherence to a prescribed therapy, such as by determining whether patients are following their care regimen and how effectively the therapy is working. This can help identify the likelihood of potential failures of patient adherence and enable healthcare providers to make the appropriate adjustments.
The successful implementation of new digital health technologies, such as connected drug delivery systems and smart inhalers, relies not only on software solutions but also on developing new measurement and feedback solutions. Such new solutions are most powerful if they can interact with the patient by collecting data, analysing it and providing feedback to the patient and their healthcare provider. This is where the right sensors provide a strong value proposition by collecting usage, safety and diagnostic data.
SENSING SOLUTIONS – WHAT ARE THE BENEFITS FOR THE PATIENT?
The nature of the interaction between a patient and a connected device comes down to the sensor. Choosing an optimal sensor solution can be challenging from a design perspective, as it must fulfil its intended function and seamlessly integrate into the patient’s user experience. On its path towards digital health, Sensirion has thus far developed multiple different sensing technologies. In the following sections, this article will focus on three of them.
Airflow Sensing
Smart inhaler add-ons are medical devices that bring additional functionality to regular inhalers by gathering information about inhaler usage. This information can easily be transmitted to a smartphone app and provide patients and healthcare providers with insights into inhaler usage. This information can contain details on the patient’s adherence to their therapy and how the inhaler is being used, such as exhaled volume, inhaled volume and a detailed inhalation flow profile containing inhaled flow rate over time, including the timing of inhaler actuation.
This feedback can allow a healthcare professional to recognise patients who adhere poorly to their therapy, or who urgently require additional training on correct inhaler use. The significance of non-adherence is highlighted by countless studies. For example, one study on in vitro lung deposition reported that patients make at least one mistake when using an inhaler as often as 70%–90% of the time, resulting in only 7%–40% of the full dose being delivered to the lung.12 Providing feedback to patients that they are using their inhaler correctly can be motivational and affirmative. Additionally, it can ease the worried minds of parents about whether or not their children are getting the correct dose delivered to the lungs.
Recording physiological data with every use of the inhaler and without interfering with the patient’s user experience is a powerful tool – recent studies have demonstrated that data relating to peak inspiratory flow or inhaled volume already provide an information base for assessing the course of the disease, enabling healthcare providers to improve its control and prevent exacerbations and hospitalisations.13 Gathering accurate physiological data on every inhalation will help to unlock many more insights and generally improve inhaler-based therapies in the future.
Airflow sensors are an essential part of smart inhalers. Sensirion’s SDP3x line of differential pressure sensors is optimised for airflow sensing in smart inhalers, allowing for sensitive and accurate measurement of airflow rates while offering other benefits for healthcare applications:
- Immediate feedback on the inhalation procedure due to direct and fast measurement.
- Meaningful feedback and improved patient confidence by accurate inhalation-to-inhalation and breath profile comparisons, enabled by high accuracy and time resolution.
- Monitoring of the course of the disease over time to help predict and prevent exacerbations by measuring lung function parameters from the recorded breath profiles.
- Seamless user experience by allowing small form factors due to the small size of the sensor and the low power consumption of its coin cell battery.
- Robust and unsusceptible to environmental conditions and user errors due to the two-port design and use of a coin cell battery.
- Enables breath-triggered drug release by providing fast response time and high sensitivity for even the smallest airflows.
Drug-Flow Sensing
Large-volume injectors (LVI) – also known as on-body delivery systems, patch pumps or wearable drug delivery devices – have been replacing conventional intravenous infusion for the delivery of large-volume doses of injectable drugs.14 These enable patients to receive precisely controlled drug dosages automatically, continuously and with minimal disruption to their day-to-day lives.
“Red biotechnology” approaches use biological materials, such as proteins and large molecules, to treat disease targets more effectively than existing pharmaceuticals and with fewer side effects. Typically, these therapeutics are expensive, viscous and highly concentrated formulations, often requiring lyophilisation and reconstitution prior to use. This means that they generally cannot be administered orally, instead requiring subcutaneous or intravenous administration in volumes of up to 50 mL. LVIs provide a simple, versatile platform for the administration of these emerging therapeutics.
In addition, LVIs have the potential to play a vital role in the delivery of precision medicine for predictive, preventative, personalised and participatory patient care. With prices and reimbursement levels based on evidence-based criteria, such therapies will likely depend on proof of patient compliance. LVIs provide a straightforward and measurable approach to ensuring adherence, thus maximising the efficacy of personalised therapies.
Controlling the drug flow rate and closely monitoring for failures is critical in automatic drug delivery systems. Sensirion’s LD20 liquid flow platform of single-use flow rate sensors offers a dedicated solution for LVIs:
- Precise measurement of the delivered drug with high accuracy is essential when a precise dosage is critical, such as with specific painkillers or chemotherapy drugs. It is also desirable with expensive, viscous or highly concentrated formulations. Accurately measuring drug flow with the LD20 technology platform enables a reliable assessment of the remaining drug inside the cartridge and can become more important as prices and reimbursement levels start to be determined by evidence-based criteria, such as proof of correct drug delivery and patient compliance.
- Detection of failure mechanisms, such as open flow and occlusion, is another valuable feature. For example, occlusion may occur for various reasons, such as tissue growth or clotting of the cannula outlet. In some cases, the patient may make a mistake with assembly of the LVI or the reconstitution of the drug, leading to an open flow condition. The remote detection of such failures assists the patient, maximises the benefits of the therapy and helps save costs by enabling remote drug administration.
- Flow range, size and interfaces are adjustable for very compact integration into the LVI due to the modularity of the LD20 technology platform. This is an essential feature to enable versatility for the drug delivery system so that it can be optimally configured to the drug type, concentration and viscosity while keeping it as compact as possible.
- Sensor compatibility with different drugs is essential, enabling the use of a single device platform for different drugs and for the reconstitution of lyophilised drugs. This feature is enabled by Sensirion’s in-house sensor calibration for one or more drugs, taking their different viscosities and properties into account.
Breath Analysis Breath analysis provides a non-invasive insight into the human body, allowing for fast, pain-free and low-cost diagnostics. Today this mainly covers gases, such as CO2, O2 and NO. Sensirion’s STC31 platform allows for the measurement of CO2 concentration in human breath, enabling entirely new applications with its low-cost technology structure, in contrast to the optical sensors that are often used in capnography applications, which are often in the lower four-digit price range. Applications in both the medical and consumer sectors can offer value to the user by providing measurement-based insight into their metabolism or fitness. The STC31 is:
- Compact and easy to fit into handheld or wearable medical devices, allowing for discrete and portable products that can be used in all environments, such as at home, outdoors or in the gym.
- A highly robust and reliable sensing solution, as there are no moving parts or sensitive optics involved.
- Manufactured without any complex assembly or alignment, further helping to save on cost and reduce form factor.
- New applications and use cases in compact and discrete portable devices that only require small batteries and allow for longer run times without the need for charging.
CONCLUSION
The 21st century has brought a number of completely new healthcare challenges – increasing numbers of chronic diseases, including cancer, COPD, asthma and diabetes; an ageing population; and broad changes to both the environment and lifestyle that amplify the occurrence, consequences and cost of such diseases. Digital health infrastructure has recently received a major push to address the healthcare challenges, automating the sharing of patient data, accessing sites of care and simplifying the payment flow.
In future, digital health will also empower patients to monitor their health from their own homes, administer their drugs without a healthcare provider present and obtain feedback on drug delivery and the course of their disease. Such functions will be implemented and enabled by portable, wearable and interactive devices that use sensors to monitor the patient. Sensirion is actively supporting this developing ecosystem with sensors built to address these needs, measuring patient airflow, drug flow and breath CO2 levels. As this journey has only just begun, Sensirion is working to improve and expand the technologies and sensors available to digital health technologies.
REFERENCES
- “Global Strategy on Digital Health 2020–2025”. WHO, 2021.
- “Noncommunicable Diseases”. WHO web page, accessed Nov 2022.
- “Ageing and Health”. WHO web page, accessed Nov 2022.
- “Global Top Health Industry Issues 2021”. PriceWaterhouseCoopers, 2021.
- Alsumidaie M, “Non-Adherence: A Direct Influence on Clinical Trial Duration and Cost”. Applied Clinical Trials, Apr 2017.
- Book D, “Exciting Partnerships Forming Between Big Pharma and Tech”. Pharmacy Times, Jan 2018.
- Ricci M, “GSK and Propeller Prepare to Launch Digital Inhaler”. PharmaPhorum, Sep 2017.
- “Adherium Releases Next Generation Adherence Monitor, SmartTurbo Model 4, for AstraZeneca’s Turbuhaler”. Press Release, Adherium, Sep 2017.
- How DiGa Fits into the Future of Digital Health”. Vertrical web page, accessed Nov 2022.
- Townsend A et al, “Experimentation to Favor Innovation: Promoting the Success of Article 51 Transformation Projects”. Therapie, 2019, Vol 74(1), pp 51–57.
- “A Guide to Remote Therapeutic Monitoring (RTM) Codes”. CareSimple, Feb 2022.
- Alt A, Pianezzi S, “How Miniaturised Liquid Flow Sensors are Revolutionising Subcutaneous Drug Delivery”. ONdrugDelivery, Issue 128 (Dec 2021), pp 44–47.
- Biswas R, Hanania NA, Sabharwal A, “Factors Determining In Vitro Lung Deposition of Albuterol Aerosol Delivered by Ventolin Metered-Dose Inhaler”. J Aerosol Med Pulm Drug Deliv, 2017, Vol 30(4), pp 256–266.
- Levy ML et al, “A Digital Inhaler Uncovering Patterns of SABA Use in Asthma and COPD”. Eur Respir J, 2020, Vol 56 Suppl 64, Article 1361.

