To Issue 143
Citation: Patel H, Holmes C, Wilson B, “Drug Delivery Platform Drives New Opportunities for Continuous-Delivery Glaucoma Treatment”. ONdrugDelivery, Issue 143 (Mar 2023), pp 34–36.
Harsh Patel, Cyonna Holmes and Brian Wilson discuss how Celanese’s VitalDose® EVA drug delivery platform can help reduce intraocular pressure by creating a desired therapeutic release profile.
“Most drug delivery systems for glaucoma aim to improve pressure management through sustained, longer-acting treatments.”
Glaucoma is a prevalent disease among older populations and the global drug market is forecast to reach around US$8.8 billion (£7.3 billion) in 2025 and around $11 billion by 2030.1 Market growth drivers include an increase in the ageing population and the emergence of new therapeutics. Although therapeutics exist to treat glaucoma, there remains an unmet need for improving treatment efficacy through increased patient adherence and drug bioavailability.
LIMITATIONS OF CURRENT THERAPEUTICS
Topical eye drops are a common first-line treatment in glaucoma. However, up to 60% of patients do not realise the full efficacy of their medications due to compliance and adherence issues, regimen complexity and, often, an initial asymptomatic presentation that results in improper dosing.2 Bioavailability, drug elimination, rapid clearance and limited absorption at target sites can also diminish therapeutic efficacy.
Treatment goals focus on reduction of intraocular pressure (IOP) by 20%–50% to provide IOP control over the lifetime of this chronic condition.3 To address this challenge, newer treatments have emerged using novel mechanisms of action (MoA) and innovative drug delivery systems and devices. While novel MoAs focus on new IOP-lowering targets, most drug delivery systems for glaucoma aim to improve pressure management through sustained, longer-acting treatments. These patient-centric delivery systems have the potential to address common unmet needs and reduce the need for frequent dosing.
“By combining these tubes, plates and shunts with a drug-eluting element, improved outcomes can be achieved with a combination device.”
SUSTAINED RELEASE FOR GLAUCOMA THERAPEUTICS
Innovative, long-acting delivery options are being developed to address current treatment limitations and different routes of administration. Ocular inserts, intracameral depots and punctal plugs represent the wide range of potential routes for sustained delivery approaches. Therapeutic release via these routes varies in duration from three months to three years and provides continuous delivery for improved IOP management.
Intracameral depots are an effective route of administration as demonstrated by Allergan’s (Dublin, Ireland) Durysta® (bimatoprost intracameral implant), the first long-acting glaucoma implant treatment to gain US FDA approval. Pipeline approaches being evaluated in trials include Ocular Therapeutix’s (MA, US) OTX-TIC (travoprost intracameral implant) and Glaukos’s (CA, US) iDose TR (travoprost intraocular implant) depots that both offer a sustained release of travoprost. These are localised dose forms placed near the site of action and are a route to consistent delivery and control of IOP.
PAIRING DRUG ELUTION WITH EXISTING GLAUCOMA DEVICES
Beyond the dose forms in development, there is an opportunity to pair ocular drug delivery with existing devices for IOP control using mechanical redirection of fluid flow. By combining these tubes, plates and shunts with a drug-eluting element, improved outcomes can be achieved with a combination device. Pairing these well-known and frequently used devices with a drug delivery mechanism would allow the mechanical and therapeutic modes of action to work in tandem. The addition of drug elution to these devices may also help reduce inflammation that is often associated with the implantation of these devices. A wide range of drugs, from antibiotics to anti-inflammatories to IOP-lowering agents, can be incorporated into these devices (Figure 1).
Figure 1: Sustained delivery of both small molecules and biopharmaceutics can be achieved with VitalDose® EVA.
Since these devices are often made of durable polymers, non-erodible polymers are a natural choice in their redesign. The strength of durable polymers, such as Celanese’s VitalDose® EVA, in ocular-based drug delivery strategies include:
- High drug loading without compromising mechanical integrity or long-term release kinetics
- Drug-release profiles of 12 months or greater for small molecule therapeutics
- Minimal risk of drug molecule instability due to the absence of polymer degradants.
The size of most drainage devices offers the potential for a large loading of one or multiple drugs to be delivered over a period of time. These parts can be made via extrusion and moulding, approaches that are typically used in the fabrication of the original device parts. Films, rods and discs represent a small sampling of the form factors that are possible for the drug delivery component (Figure 2).
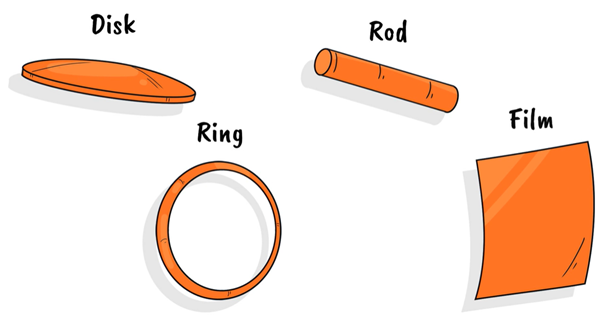
Figure 2: Form factor options provide flexibility in design.
These delivery geometries can work to replace existing segments or entire parts within a medical device to allow for a dual-action approach. IOP-lowering medications can be released from VitalDose® EVA and the loading percentage can be tuned to achieve the desired release profile. Additionally, different form factors (film, rod, etc.) have demonstrated sustained release that further highlights adaptability to current medical devices.
REFERENCES
- “Ophthalmology Drugs Global Market Opportunities and Strategies to 2030: COVID 19 Impact and Recovery”. The Business Research Company, May 2020.
- Zaharia A et al, “Adherence to Therapy in Glaucoma Treatment – A Review”. J Pers Med, 2022, Vol 12(4), p 514.
- Rahic O et al, “Novel Drug Delivery Systems Fighting Glaucoma: Formulation Obstacles and Solutions”. Pharmaceutics, 2021, Vol 13(1), p 28.

