Citation: Gupta S, “Drug Delivery to the Eye: Overcoming the Challenges of Intravitreal Prefilled Syringes”. ONdrugDelivery, Issue 118 (Apr 2021), pp 18–21.
Suresh Gupta discusses the challenges of developing a PFS product for intravitreal injection, highlighting how designing for the intravitreal space, rather than the standard parenteral, imposes a number of additional requirements on the design.
In the field of ophthalmic drug delivery, intravitreal injections using prefilled syringes (PFSs) are becoming increasingly prevalent – improving patient treatment and outcomes by delivering an efficacious shot of medicine directly into the eye. Opportunities abound in this still-evolving area of ocular therapy innovation, but significant challenges remain. For example, a surface-level examination might make adapting the design of a standard parenteral PFS for intravitreal use seem relatively straightforward. However, that’s a long way from the reality.
“A surface-level examination might make adapting the design of a standard parenteral PFS for intravitreal use seem relatively straightforward. However, that’s a long way from the reality.”
This article explores the theme of delivery to the eye, a growing area of interest in ophthalmology that spans drugs, implants and gene therapies. It will unpack the challenges of PFS development, drawing on the author’s professional experience to reveal how innovators can best begin to plot a path to success.
To begin with, let’s consider PFSs, first used by healthcare professionals and patients for parenteral (administered anywhere other than the mouth and alimentary canal) injections under the skin or into the muscle. It is only in recent years that PFSs have made their way into the field of intravitreal injections. These injections deliver drugs directly into patients’ eyes for the treatment of conditions such as wet age-related macular degeneration (AMD), diabetic retinopathy and diabetic macular oedema. Examples include Genentech’s Lucentis® (ranibizumab) and Regeneron’s Eylea® (aflibercept), both anti vascular endothelial growth factor (VEGF) injections that come in a ready-to-use PFS.
At first glance, it might look as if the same PFS design has found its way into the intravitreal injection space. But the devil is in the details. Together with colleagues, the author has made the PFS journey from standard parenteral to intravitreal application. So, let’s highlight some of the key differences and challenges when it comes to PFSs for intravitreal injections, compared with designing for the standard parenteral space, and discuss some potential improved solutions based on publicly available information.
TACKLING THE ISSUE OF STERILITY
“A key difference between an intravitreal and standard parenteral PFS is that the former must be sterile both inside and outside, whereas the latter does not have to be sterile on the outside. What might sound like a simple additional requirement poses a tremendous challenge to the design, manufacture and packaging of an intravitreal PFS.”
Endophthalmitis, an inflammation of the interior cavity of the eye most commonly caused by an infection, is a major concern associated with intravitreal injections. It poses a significant challenge with respect to maintaining the sterility of the clinical environment in which the injection takes place, and also in terms of how the sterility of the PFS and its contents is achieved and maintained throughout manufacturing, shipment, storage and use.
A key difference between an intravitreal and standard parenteral PFS is that the former must be sterile both inside and outside, whereas the latter does not have to be sterile on the outside (Figure 1). What might sound like a simple additional requirement poses a tremendous challenge to the design, manufacture and packaging of an intravitreal PFS. This challenge spans the syringe material that protects the contents from the ambient air; the design that enables aseptic filling of the drug content and preserves it during shipment, storage and use; the packaging that allows terminal sterilisation and ease of unpacking without compromising sterility; and the design that facilitates ease of use and reduces use errors.
Figure 1: PFSs for intravitreal injection must be sterile both inside and outside to avoid risk of endophthalmitis.
ENSURING DOSE ACCURACY
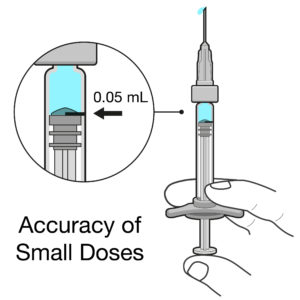
Figure 2: Intravitreal injections deal with doses of 50 μL or less, so even very small errors in dose can have significant impact.
Intravitreal injections are typically very small doses of 50 μL or less, equivalent to a drop of liquid. When you compare this dose with a 1 mL standard parenteral PFS, the delivered volume is 20 times smaller. Any slight deviation from the intended dose could result in a significant underdose or overdose in terms of both percentage and therapeutic effect (Figure 2). For example, if there is an error of 20 μL for a 1 mL standard parenteral PFS, it would cause a 2% inaccuracy in the dose, whereas the same error in an intravitreal injection would cause a 40% underdose or overdose – a big difference!
An underdose of such magnitude for an intravitreal injection may mean a compromised therapeutic effect – or no therapeutic effect at all – whereas an overdose into an eye, which is roughly 20 mm in diameter, could cause detrimental intraocular pressure (IOP) and, in rare cases, drug toxicity and other complications.
So, what are the design challenges when it comes to achieving a dose accuracy with an intravitreal PFS? The answer can be found by first understanding the sources of dose inaccuracies. There are a number of possibilities:
- Tolerances in the bore diameter of the syringes
- Inconsistencies in the shape of the neck of the syringes
- Irregularities in the geometry of the stoppers
- Unpredictability of the dead volumes of the syringes and needles.
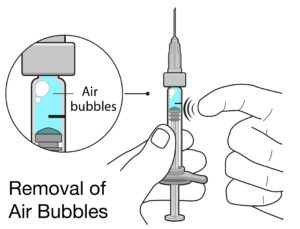
Figure 3: Air bubbles in PFSs for intravitreal injection can cause complications, and can take up space inside the syringe that significantly lowers the delivered dose.
The other most likely source is the usability of the product. One such issue could be a user’s inability to remove any air bubbles and align the stopper precisely with the dose mark (Figure 3). Therefore, the solution lies in the manufacturer’s ability to minimise or eliminate the sources of inaccuracies, compensate for any residual potential inaccuracies (e.g. over-filling of the PFS) and incorporate thorough human factors engineering into the development process.
Even when all the design- and manufacturing-related sources of inaccuracies have been addressed or compensated for, it is still difficult to predict and control users’ behaviour. Will they decide to align the tip of the stopper with the dose mark? Or will they align the mark with one of the ribs on the stopper? Or maybe they use the flat bottom of the stopper as it looks like a more defined reference point. These are some of the genuine behaviours exhibited by experienced ophthalmologists and retina specialists.
Needless to say, if one aligns the bottom of the stopper with the dose mark with some of the marketed intravitreal PFSs, they are unlikely to deliver any drug at all, without even realising it. Imagine this from the patient’s perspective; they have undergone all the pain and inconvenience, not to mention the risks, of an intravitreal injection for nothing. To top it off, they may be self-funding their expensive treatment (or, in this case, non-treatment) and their condition may deteriorate further as a result.
DEALING WITH BUBBLES AND FLOATERS
“There are many opportunities for significant use errors – not removing the air bubbles, not setting the dose accurately and failing to maintain sterility during use. All of these errors could result in harm to patients and compromise their treatment considerably.”
A PFS injection containing small air bubbles delivered into subcutaneous tissues may not have much effect on the patient. But an injection of air bubbles into a patient’s vitreous cavity may cause complications. This is another important difference between a standard parenteral and intravitreal PFS products. Although literature suggests that an injection of small air bubbles into an eye is likely to get resorbed within a couple of days, it may affect vision temporarily due to bubble-related floaters. Large air bubbles, on the other hand, could result in a high IOP and related complications.
Another concern with air bubbles is that they tend to stick to the syringe wall and get trapped within the dead spaces, making them hard to expel during priming of the syringe. These bubbles then take up a significant proportion of the 50 μL volume, and therefore compromise the actual amount of medication delivered to the eye. In the worst case, air bubbles, if drawn from the ambient air during priming (design allowing), may result in an infection of the eye – one reason why the Lucentis® PFS plunger rod is not attached to the stopper.
Besides air bubbles, other particulates, such as droplets from the lubricant (usually silicone) used to facilitate smooth stopper movement and fine particles that have rubbed off from the stopper or syringe wall, may also cause floaters and other complications in a patient’s eye. On the other hand, most of these may have no effect or cause only a minor local irritation to the skin with a standard parenteral injection.
So, what are the design challenges and the solutions? The following are all key to the success of an intravitreal PFS:
- The selection of the syringe, plunger, cap material and a state-of-the-art manufacturing process (for example, the low siliconisation achieved by Lucentis®) to reduce particulates
- Using bubble-free filling technologies
- Ergonomic design of the user-interface
- Effective instructional materials tested and validated via human factors studies.
IMPROVING USABILITY AND EFFICIENCY
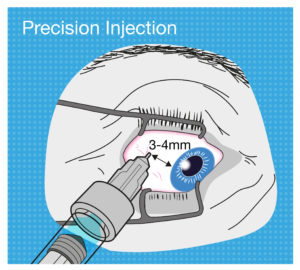
Figure 4: The tolerance for use error when performing an intravitreal injection is very low, so intravitreal PFS design should aim to be as ergonomic as possible.
The likelihood of potential use errors and their consequences are greater with an intravitreal injection compared with a standard parenteral injection. For example, if a user inserts the needle of a subcutaneous PFS into the skin at a 60° angle as opposed to the intended 45°, it might hurt the patient a bit more or – in the worst case – compromise the injection’s therapeutic effect to a very small degree.
But now let’s think about the needle insertion into the eye for an intravitreal injection. It has to be precisely 3–4 mm away from the limbus towards the centre of the globe, or the needle may damage the lens or other internal tissues. As another example, if the user’s hand moves whilst depressing the plunger with a standard parenteral PFS, the patient might feel a bit more pain or discomfort, or it may cause a minor laceration. But if this happens with an intravitreal injection, the consequence could be extremely severe (Figure 4).
There are many opportunities for significant use errors – not removing the air bubbles, not setting the dose accurately and failing to maintain sterility during use. All of these errors could result in harm to patients and compromise their treatment considerably.
Another important point of note from an ophthalmologist’s or retina specialist’s perspective is the efficiency of the use process. These experts perform a large number of intravitreal injections per day. Therefore, even a small reduction in time per injection would save them a significant amount of time overall, hence increasing their throughput.
Although it might appear that an intravitreal PFS has simple ergonomics and room for improvement in this area is limited, the application of a thorough human factors engineering process, coupled with a robust risk management system, will identify any potential use errors, appropriate mitigations and opportunities for improvement. The process focuses on understanding the users’ needs (both obvious and latent), considering how subtle alterations to the user-interface could cater to those needs, and designing out problems through an iterative process of evaluation and improvement. All this makes the product safe and effective, with the addition of increased user satisfaction.
LOOKING BEYOND INTRAVITREAL PFSs
It is fair to say that intravitreal PFSs currently on the market have tackled some of the many challenges very well. But it’s important to remember this: with a rich domain knowledge and critical design thinking, intravitreal PFS design can be improved further, as some challenges still persist. Moreover, even if a new manufacturer wants to achieve only what these marketed products have achieved, they must consider the intellectual property (IP) around some of the design solutions. It is a narrow design space fraught with challenges.
Maybe the next step is to look beyond the use of a PFS, and also outside the intravitreal space. The science is evolving and so too have some of ophthalmic treatment methods and approaches. For example, some of sources suggest that biodegradable and non-biodegradable implants are an effective way of providing sustained drug delivery to eyes, thereby improving the treatment outcomes. Perhaps it’s also time to look beyond intravitreal drug delivery to suprachoroidal (the space between the sclera and choroid that traverses the circumference of the posterior segment of the eye) and subretinal (beneath the retina) injections, which focus on providing localised delivery of existing treatment options and of new gene and cell therapies, improving outcomes and minimising side effects. These new methods require sophisticated, but not necessarily complex, delivery systems. As the technology advances, we will certainly see more treatment options for patients as well as great opportunities for device manufacturers.
Dr Gupta and his colleagues have a wealth of experience in the subtle design challenges and IP complexities in the intravitreal drug delivery space, and a keen understanding of the various needs involved, and would be happy to discuss the topic in more detail.

