Citation: Ventura Fernandes J, Villax P, “Dry Powder Inhalers: Towards Effective, Affordable, Sustainable Respiratory Healthcare”. ONdrugDelivery Magazine, Issue 102 (Nov 2019), pp 5-8.
João Ventura Fernandes and Peter Villax look at the trends shaping the development of dry powder inhalers, highlighting the need for a shift in industrial strategy towards effective, globally affordable, sustainable devices.
“Without major technological breakthroughs in finding alternatives which are not greenhouse gases, pharmaceutical companies may lean towards the use of alternative inhaler technologies.”
Inhaled drug delivery is established as the primary choice for airway disease treatment and continues to hold high potential for systemic drug delivery. Since its invention in the 1950s, the pressurised metered dose inhaler (pMDI) has been the inhaled therapy “gold standard”1 for airway diseases such as asthma, as a result of being easy to use, multi-dose and inexpensive to manufacture.
However, its drug delivery performance remains dependent on patient co-ordination – often leading to high drug losses – and environmental concerns have emerged with respect to the use of propellant-driven pMDI technology. Although initial chlorofluorocarbon (CFC) propellants have been discontinued due to their impact on the ozone layer, their hydrofluoroalkane (HFA) replacement propellants are unfortunately potent greenhouse gases – 2,000 times more potent than carbon dioxide.1
The global battle against climate change arising from greenhouse gas emissions may drive regulators to subject pMDI technology to carbon emission restrictions. Without major technological breakthroughs in finding alternatives which are not greenhouse gases, pharmaceutical companies may lean towards the use of alternative inhaler technologies.
In this regard, an alternative available now is the propellant-free, breath-actuated dry powder inhaler (DPI). In terms of total lifecycle harmful gas emissions, DPIs not only produce 10 times less greenhouse gas emissions than pMDIs2 but can also achieve lung delivery efficiencies and deliver drug loads which are beyond the reach of pMDI technology. DPI market entry occurred in the 1960s and market adoption by pharma companies has been growing steadily as a result of these advantages. The DPI product market size surpassed US$16 billion (£13 billion) in 2015 and is expected to continue growing at 5% CAGR until 2024.3 A total of 114 inhaled products are currently being marketed,3 delivered to patients by about 49 million DPI devices produced per year.3
TRENDS SHAPING DPI DEVELOPMENT
Currently, 100 new inhaled drugs in the pharma development pipeline have already opted for DPI technology and are expected to achieve an ever-growing market share for this category of device. Looking ahead, as a result of evolving industrial and societal trends, four key requirements are expected to shape DPI developments, as shown in Figure 1:
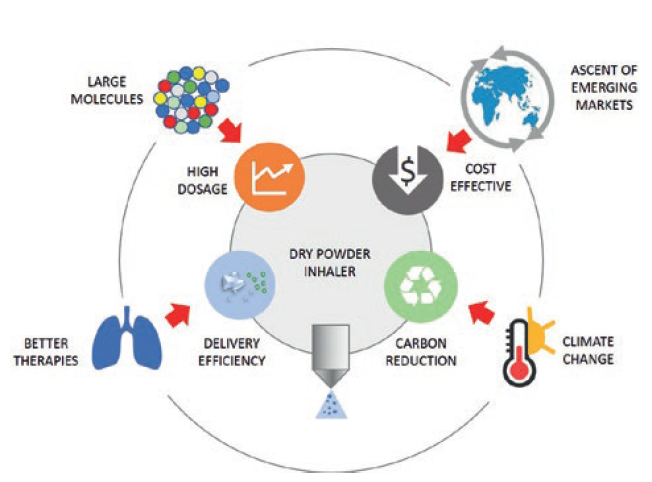
Figure 1: Trends and requirements shaping dry powder inhaler development.
- Delivery efficiency. To deliver the drug as efficiently as possible to where it needs to go in the airways remains the number one goal. Developers will continue their drive to improve drug delivery efficiency to the lung through reduction of losses in the device and the upper airways as this enhances patient treatment. Recent inhaler developments have reached lung doses between 40% and 50% as a percentage of label claim using a number of formulations: traditional carrier-based; carrier-based with force control agents; and carrier-free formulations.4
- High-dose drug delivery. There are new large molecules entering the respiratory development pipeline of large pharma and biotechs – namely proteins, oligonucleotides, antibodies and nanobodies. As a result, new DPIs may be required to deliver higher doses using drug-rich, carrier-free formulations, using large powder volumes and possibly requiring multiple patient inhalations.
- Cost effectiveness at global scale. It is forecast that, by 2030, seven of the 10 largest economies by gross domestic product in purchasing power parity will be emerging markets – China, India, Indonesia, Turkey, Brazil, Egypt and Russia.5 The ascent of these countries will provide DPI growth opportunities for cost-leader inhalers to harvest on a global scale.
- Environmental sustainability. Today’s inhalers are contributing a significant carbon footprint,2 and public opinion and regulatory pressure is already pushing towards environmental improvements.6 The ideal environmentally friendly inhaler is a simple, effective, propellant-free device made of only a few recyclable-material parts, thus minimising its full environmental lifecycle cost.
SHIFTING THE INDUSTRIAL PARADIGM
“Currently, 100 new inhaled drugs in the pharma development pipeline have already opted for DPI technology3 and are expected to achieve an ever-growing market share for this category of device.”
In DPI development, the innovator strategy has been to develop new, more effective molecules to treat airway diseases delivered by ever-more complex and expensive DPIs, as a means to increase development and cost barriers for generic competition. It is now common that state-of-the-art multi-dose DPIs are made from more than 20 plastic parts, which the patient uses for two months and then discards and replaces by a new unit.
Such an industrial strategy is successful in the market but results in a major consumption of primary raw materials and energy – and these account for carbon emissions and generate large quantities of plastic waste that needs further processing to avoid impacting natural ecosystems.
In fact, Figure 1 underlies the need for DPIs to be fundamentally simple to use, so that the patient is able to understand how they work and to use them correctly to achieve effective delivery – and made of only a few recyclable-material parts, so that both its environmental footprint and overall cost of goods are the lowest possible. This requires a shift in industrial strategy towards effective, globally affordable, sustainable DPIs. Hovione Technology aims to realise this paradigm shift with the portfolio of DPIs shown in Figure 2.
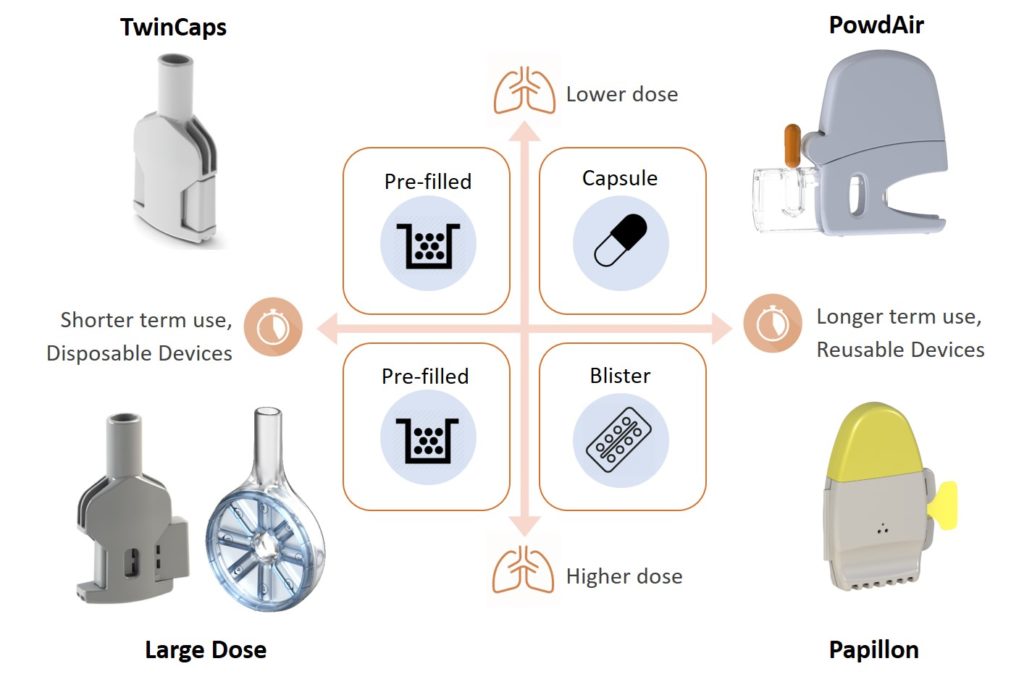
Figure 2: Hovione Technology’s portfolio of innovative dry powder inhalers.
SIMPLE AND HIGH PERFORMING
Unlike most other medical devices, inhalers do not need to be fundamentally complex and built from many parts to achieve a high and robust performance in drug delivery to the lungs. An example is the TwinCaps DPI (Figure 2), launched in Japan in 2010 as part of Daiichi-Sankyo’s Inavir (laninamivir) influenza drug product. Its extreme simplicity of operation, requiring only one step per inhalation, and its ease of manufacture – two plastic parts only – were key to its commercial success.
Inavir currently has the largest market share in the Japanese influenza market and TwinCaps has become the world’s largest-selling single-use-dose disposable inhaler. In a recent study of aerodynamic performance with an asthma/ COPD carrier-based formulation, TwinCaps demonstrated high and robust drug delivery performance across different inspiratory flow rates and inhalation volumes.7
As shown in Table 1, the TwinCaps delivered dose of more than 90% of total dose and fine particle dose of over 40%, substantially independent from patient-generated inspiratory flow rate and inhaled volume, indicates that performance-leading DPIs can be simple in design, construction and operation.
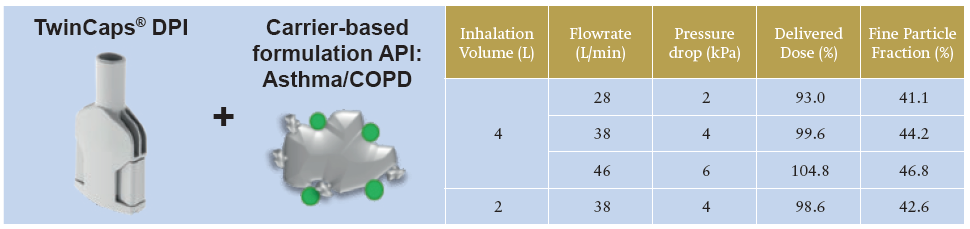
Table 1: TwinCaps DPI – delivery performance with an asthma/ COPD drug carrier-based formulation at two flow rates and two inhalation volumes.7
ENABLING HIGH DOSE
When it comes to inhaled delivery of the large molecules entering the drug development pipeline – which are expected to require large lung doses – not only innovative formulations are needed but also new and effective large-dose inhalers, tailored to the specific usability and convenience needs of patients. As shown in Figure 2, Hovione Technology is developing such large-dose DPIs.
These include 8Shot, the world’s first eight-dose, factory-filled DPI enabling delivery to the lungs of therapeutic doses up to 400 mg in eight inhalation manoeuvres. In use, the patient just turns a dose wheel to access the next unit dose. 8Shot provides a patient-friendly solution for high-dose delivery without the burden of instructing the patient to reload an inhaler multiple times with a capsule or blister, leading to a long, time-consuming, error-prone use sequence.
As shown in Figure 3, the 8Shot DPI is designed to allow the patient to sequentially inhale the right amount of drug or particle-engineered powder formulations, mitigating the coughing risk resulting from sudden airways exposure to a single large dose. Being manufactured from only two plastic parts turns this large-dose DPI into an economically viable option from short to long-term inhaled treatments requiring high-dose delivery.
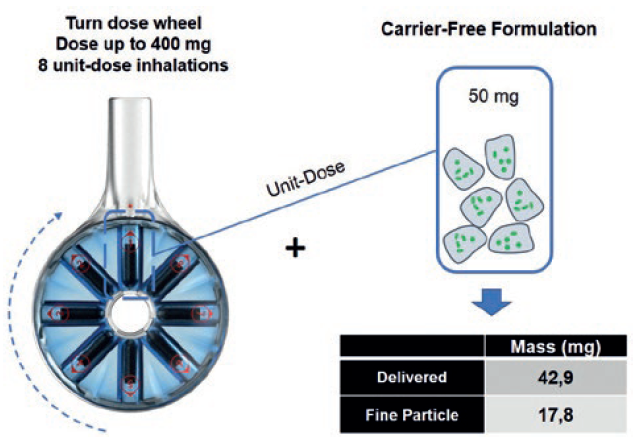
Figure 3: The 8Shot DPI, the world’s first eight-dose factory-filed inhaler – enabling high-dose drug delivery to the lungs. In this example, up to 140 mg of carrier-free drug powder may be deposited in the lung.
GLOBALLY AFFORDABLE, SUSTAINABLE CARE
When aiming to fulfil the need for readily accessible and affordable treatments across world regions and delivering environmental gains, the DPI developer faces a question – is it worth spending tens of millions scaling up a 20-part DPI device only used for a few months or is it preferable to choose a device that costs at least 10 times less to bring to production, with less environmental impact and without compromising delivery performance?
The Papillon DPI (Figure 2, bottom right), Hovione Technology’s new single-dose, reusable blister-based DPI, unlocks a paradigm shift for pharma companies developing inhaled drugs. On account of it being made from a single, reusable plastic part, as shown in Figure 4, Papillon can achieve unit dose costs competitive to multi-dose inhalers at a fraction of the development cost and risk associated with complex 20-part inhalers.
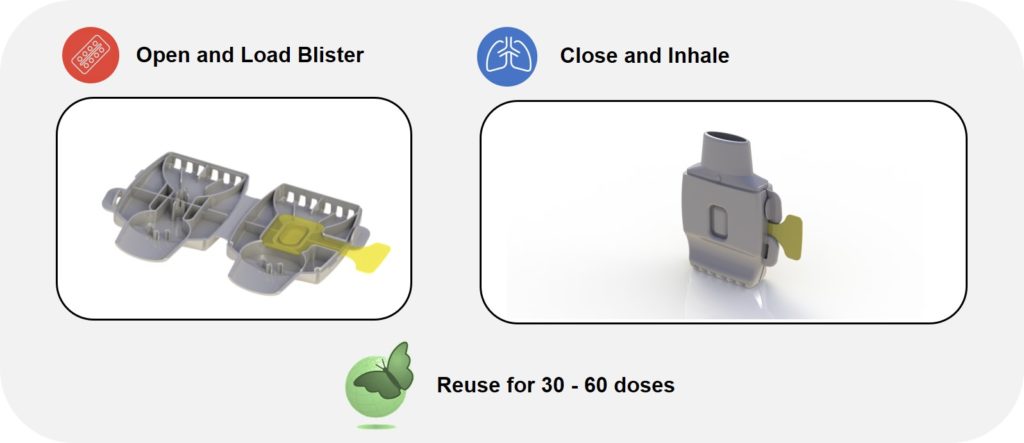
Figure 4: The Papillon DPI – a single-dose, reusable blister-based DPI built from a single part.
The Papillon inhaler delivers extreme ease of use: the patient loads the blister, closes the device and inhales the dose. Papillon may then be reused for the remaining doses provided (Figure 4). Its patented system for automatically piercing the loaded blister enables the single-part DPI construction. Furthermore, it features a large blister size for accommodating a wide range of inhaled drug doses. Papillon is designed for global, industry-leading inhaler affordability across emerging and established markets.
CONCLUSION
We have presented the case for simple, inexpensive DPIs with a reduced number of parts. A growing preference from pharma companies for DPIs delivering new drugs under development is supported by their delivery efficacy and ease of use – and, in the case of the devices presented in this article, large-dose capability, ease of manufacturing and better environmental sustainability. Hovione Technology’s portfolio of DPIs – disposable, capsule-based, blister-based and large-dose inhalers – addresses the need for effective, globally affordable and increasingly more sustainable inhaler technologies.
REFERENCES
- Myrdal P, Sheth P and Stein S, “Advances in metered dose inhaler technology: formulation development”. AAPS PharmSciTech, 2014, Vol 14, pp 434–55.
- Wilkinson A and Mortimer F, “Sustainable respiratory healthcare: environmental impact of inhalers”. Respiratory Drug Delivery Europe, 2019, Vol 1, pp 137–148.
- “2019-2024 Global Dry Powder Inhaler Device Market”. Market Study Report LLC, 2019.
- Boer A et al, “Dry powder inhalation: past, present and future”. Expert opinion on drug delivery, Vol 14(4), pp 499–512.
- The biggest economies by 2030. Standard Chartered. March 2019
- Lynn E, “Asthma drugs: industry and government look for greener methods of delivery”. BMJ 2019, pp 365–4135.
- Cruz C et al, “Carrier-based formulation dry powder inhalers using TwinCaps®: aerodynamic particle size distribution and laser diffraction analysis for a typical patient population peak inspiratory flow rate range”. Proc Respiratory Drug Delivery Europe, 2019, pp 277-282.

