To Issue 168
Citation: Cox B, Grant T, Zaki L, “Elevating Digital Patient Support: Nothing Matters Unless it Changes Behaviour”. ONdrugDelivery, Issue 168 (Jan 2025), pp 56–59.
Ben Cox, Thomas Grant and Lara Zaki discuss the role of behaviour design in onboarding for self-injection devices and how a robust and balanced framework can enhance the patient experience, reduce injection errors and promote safe and effective use.
“This surge in demand for injectable therapies has dramatically shifted the patient landscape, with many individuals who may have never considered self injecting now facing the task of regularly administering their own medication.”
Patient-use disposable autoinjectors are widely used for convenient drug delivery. Moreover, smart injection devices and their surrounding digital health applications can be effective tools to enhance patient engagement and adherence to treatment regimens, ultimately ensuring the best health outcomes. Most recently, the rapid rise of GLP-1 receptor agonists, originally developed for diabetes and now widely prescribed for obesity, has introduced millions of new patients to self-injection for the first time. This surge in demand for injectable therapies has dramatically shifted the patient landscape, with many individuals who may have never considered self-injecting now facing the task of regularly administering their own medication.
Understanding the role of behaviour design is fundamental to developing solutions that will offer tangible benefits and create real impact for patients. To develop effective connected devices and digital patient support materials, efforts should be focused on specific decision points in the user journey that have demonstrable impact on long-term engagement. The overarching user experience (UX) strategy, as well as the individual features of a product, must prioritise a set of target user behaviours that make a difference, while also respecting and complimenting the existing patient journey. This requires an understanding of user capabilities and motivations, as well as the potential barriers to achieving the desired outcome.
THE REALITY OF PATIENT ONBOARDING
Despite the growing prevalence of these devices, there are numerous challenges related to onboarding and use-related injection errors. It is known that access to training and education varies across patients, and that patient anxieties, learnings and expectations around device use create opportunities for injection errors to occur.
Most product labels outline the necessity for patients or caregivers to administer injections under the supervision of a healthcare professional (HCP), who should assess their suitability and willingness to self-administer injections, as well as provide instruction on proper injection technique. Despite this stipulation, 40% of HCPs acknowledge that they do not provide any training to patients on the use of injection devices.1–3 In the many cases where manufacturers do not provide a training programme to onboard new users onto a self-administration therapy, it is down to the HCP’s judgement on what level of training is deemed adequate. Furthermore, HCPs are not always trained on the devices themselves, with a survey reporting that 43% of HCPs have not received training on specific device use.1
Given these training inequalities, there is a need for accessibility, comprehensiveness, individualisation and emotional support when onboarding patients to a device. The prevailing aim for all device manufacturers should be to set their patients up for success – not only for new patients using a device for the first time but also those who are experienced and switching to a new device. There are very real risks that patients can be lost in the first weeks and months of using a device due to poor onboarding or user experience. The question is, what approaches can be taken to improve onboarding and long-term engagement?
BEHAVIOUR DESIGN
Behaviour design facilitates the development of products and interventions that are informed by behavioural and cognitive science principles. When applying behaviour design models, the aim is to design products based on an understanding of how people make decisions, behave and interact with the environment. This ensures that products encourage engagement in desired behaviours as much as possible.
To create products that are informed by applied behavioural science, it is crucial to adopt a behaviour design framework that ensures a systematic and balanced approach (Figure 1). The framework should integrate behavioural science principles with UX design, human factors engineering (HFE) and iterative testing methods. There are three core phases to consider – foundations, behavioural science and iterative design. Together, these represent a series of iterative and interconnected activities that streamline the design of connected devices and digital patient support materials.
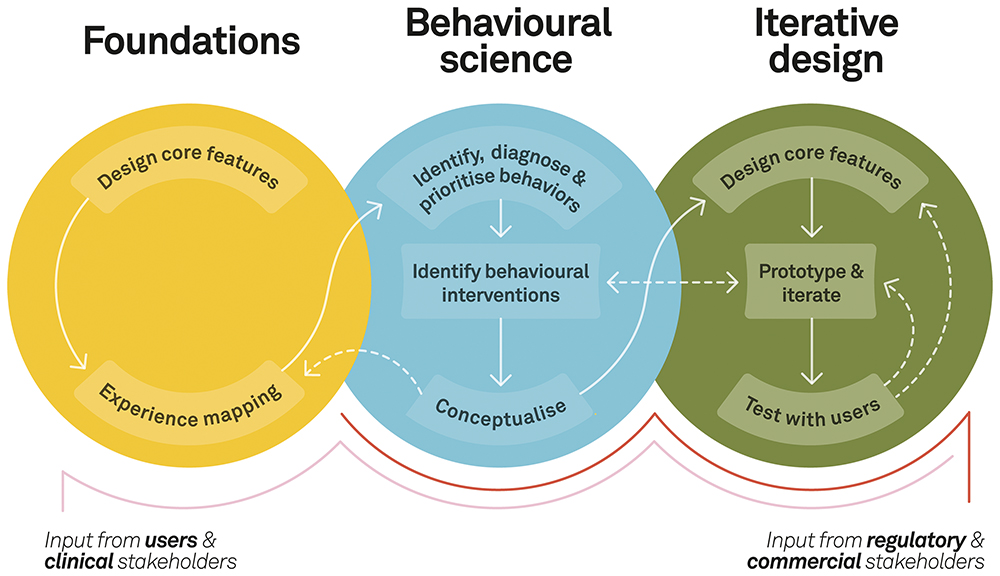
Figure 1: A behaviour design framework for a balanced approach.
Phase 1: Foundations
Foundational research establishes a robust understanding of the patient experience in the context of the broader healthcare ecosystem. By combining exploratory research and experience mapping, it is possible to capture the standard of care, existing workflows and a detailed, evidence-based view of the patient journey. For instance, when examining challenges in training and onboarding, it is essential to define what a gold standard, HCP-led injection training session entails.
“It is equally important to understand the patient’s perspective – their current experience, pain points and the tools and techniques they rely on.”
It is equally important to understand the patient’s perspective – their current experience, pain points and the tools and techniques they rely on. This research should go beyond internal factors related to device use and encompass the surrounding and external influences that shape patients’ learning experiences as they first engage with self-injection. Depending on the information available, these insights can be gathered through a combination of semi-structured interviews, observational studies, analysis of previous validation studies, post-market surveillance and literature reviews. The research should be designed to systematically build an understanding of the circumstances that influence behaviours related to device interactions. Learnings can then be translated into a comprehensive experience map, highlighting clinical touchpoints, tools, pain points, emotions and decision points.
Phase 2: Behavioural Science
The foundational research can then be analysed through the lens of a behavioural science model, such as the COM-B model of behaviour developed at University College London.4 The COM-B model is recommended in the development of connected medical devices and digital patient support materials as it provides a structured approach to understanding target behaviours and identifying relevant evidence-based interventions.
The first step of COM-B is to translate the research insights into prioritised target behaviours that have the greatest potential impact on clinical outcomes and are therefore the most relevant to address with the digital solution. These should be specific, measurable behaviours that contribute to patient engagement and adherence. These behaviours are then “diagnosed” based on data in the experience map.
In the COM-B model, a behaviour is defined as a culmination of factors relating to capability, opportunity and motivation. By identifying barriers related to these three factors, development teams can better understand where to focus digital interventions. For example, the foundational research may highlight that failure to wait for medication to reach room temperature increases pain perceptions and affects drop-off rates. The barriers preventing users waiting sufficiently may include both opportunity-related barriers, such as time constraints, and capability-related barriers, such as lack of knowledge of the consequences.
The COM-B model includes a taxonomy of behaviour change techniques (BCTs) and a guide on where they have shown success. Self-monitoring of behaviour, prompts/cues and information about consequences are examples of BCTs that are linked to specific behavioural barriers. By applying this approach, development teams can select BCTs based on literature citations, implementation feasibility and applicability to the specific user demographic. These are not prescriptive and instead provide a starting point that helps to de-risk the innovation process and enable concept development that is informed by evidence. For example, BCTs and concepts could be related to specific mediums of educational material, informative packaging, connectivity features or companion apps.
Phase 3: Iterative Design
Once concepts have been generated, they can be iteratively designed, prototyped and tested. It is important to design specific features that bring the concepts to life, to test whether they have the intended impact with the specific patient demographic. Taking the example of patient training and onboarding, digital educational materials might include a video with a specific narrative. When working on a video concept, the first step is to break down the content into a storyboard of scenes and topics that need to be covered and to work as a cross-disciplinary team to embed the BCTs identified into the design. In this case, BCTs might include providing information about consequences to demonstrate why certain behaviours are important, whilst applying positive framing. Demonstration and self-monitoring techniques can also be incorporated through video voiceover and narrative, for example, as a dialogue between a patient and an HCP.
If a concept in development is a smart device with a connected app, the features should reflect specifically what kind of data will be useful for patients to monitor, what language should be used to present the data in the app and what kind of feedback patients need from the device. This can be informed by the BCTs and behavioural barriers established earlier in the framework. A blueprint of the digital product can then be outlined to demonstrate how behavioural techniques can be embedded into the UX.
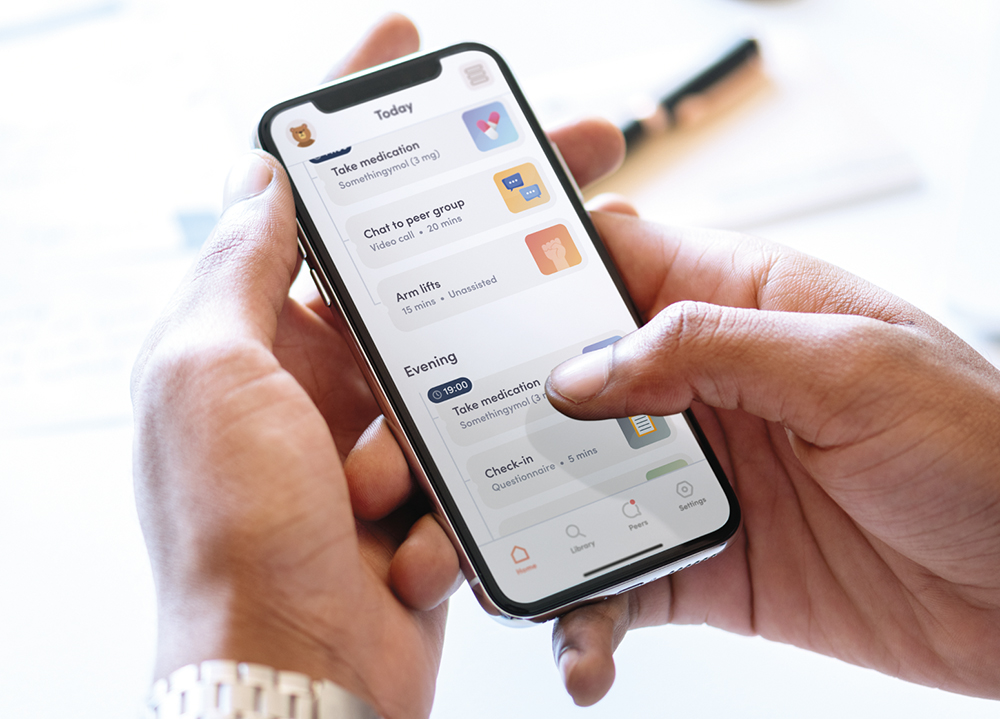
Figure 2: A functional mock-up of a digital companion app.
Rapid prototyping techniques can be used to enable iterative refinement of product features with feedback from clinical and regulatory experts, as well as representative users, through functional, interactive code-free mock-ups of the digital interface (Figure 2). This process allows developers to validate screens and components to ensure they are being understood as intended.
“When creating digital educational materials, it is recommended to conduct multiple rounds of user testing with patients and HCPs, evaluating various learning methods and comparing designs against the gold standard of hands-on injection demonstrations.”
When creating digital educational materials, it is recommended to conduct multiple rounds of user testing with patients and HCPs, evaluating various learning methods and comparing designs against the gold standard of hands-on injection demonstrations. This approach allows for a thorough assessment of objectives related to injection performance, engagement, confidence and user experience, using a combination of qualitative feedback and performance data to build evidence.
Incorporating Stakeholder Input
Throughout the application of the behaviour design framework, the product design needs to be evaluated against clinical, regulatory and commercial requirements. Continuously consulting with subject matter experts and following a risk-based process can help to de-risk the development and result in a digital product that is safe and effective, but also compliant and marketable.
CONCLUSION
The continuous rise in self-injecting patient populations underscores the need for thoughtful behaviour design that enhances patient experiences, reduces injection errors and promotes the safe, effective use of therapies. Improved onboarding and tailored digital support can play a transformative role in helping patients feel confident, minimising barriers and supporting long-term adherence to treatment. As healthcare adapts to this shift, the importance of patient-centric design and comprehensive onboarding has never been greater.
Despite the benefit these approaches can bring, connectivity and digitisation of patient support materials should not be an example of “technology push” and may not be the best solution for all use cases. Combining behavioural science with robust HFE and UX design practices can guide the selection of evidence-based principles, which can then be translated into concrete UX strategy, guidelines and features.
Ultimately, a behaviour design framework offers a structured approach to developing solutions, making the most of what digital devices and tools have to offer. By de-risking digital device development within the constraints of a highly regulated industry, adopting a balanced framework can streamline innovation and maximise the success of connected devices and digital patient support materials, driving meaningful advancements in patient care.
REFERENCES
- Lang VA, Nalan D, “Combination Product Patient Training: How Are Patients Trained and Who Conducts the Training?”. 2018 Design of Medical Devices Conference (Minneapolis, MN, US), Apr 9–12, 2018.
- Schiff M et al, “Chronic Disease and Self-Injection: Ethnographic Investigations into the Patient Experience During Treatment”. Rheumatol Ther, 2017, Vol 4(2), pp 445–463.
- Hawthorne J et al, “The current paradigm for biologic initiation: a confirmatory quantitative analysis of self-injection training practices”. Expert Opin Drug Deliv, 2022 Vol 19(6), pp 733–742.
- Michie S, van Stralen MM, West R, “The behaviour change wheel: a new method for characterising and designing behaviour change interventions”. Implement Sci, 2011, Vol 6, article 42.

