To Issue 149
Citation: Kaiser-Kershaw S, “Empowering Patients for Self-Care with NFC Connectivity.” ONdrugDelivery, Issue 149 (Jun 2023), pp 20–24.
Sylvia Kaiser-Kershaw looks at how near-field communication technology can be used to add connectivity to medical devices, empowering patients to self-administer medication.
Over the past several years, specialty pharmaceuticals and other biotech products aimed at treating the increasing occurrence of chronic diseases, such as diabetes, cardiovascular disease and chronic obstructive pulmonary disease, have dominated pharma profitability, new product filings and sales revenue. At the same time, there has been an increased focus on self-care at home, with patients administering treatments themselves and – increasingly – receiving injectable and inhalable drugs directly, without having to visit a doctor’s office or hospital.
“Digitisation can make drug delivery devices more intelligent, improve patient outcomes and offer clinicians a better remote-care relationship with patients.”
Many of these self-administered drugs are supplied in connected drug delivery devices, which make it possible to create a valuable link between the patient and the medication, and between the healthcare provider and the patient. Digitisation can make drug delivery devices more intelligent, improve patient outcomes and offer clinicians a better remote-care relationship with patients. Smart injectors can prevent the use of expired, wrong or counterfeit drugs. Smart inhalers can send notifications to a smartphone to remind patients they have not taken their medicine for the day. Smart devices can track dosage and delivery time to ensure patients comply with their regime and even keep doctors informed. Intelligent digital solutions can thus provide a differentiation for pharma companies, improve the patient experience and therapy outcome, and assist clinicians in their daily work.
Connected drug delivery devices are available in single-use and reusable formats, and either integrate connectivity directly into the device or use connectivity as an add-on to a mechanical device. All these formats are evolving rapidly, and demand is projected to remain quite strong in the coming years. In its recent report on connected drug delivery devices, Mordor Intelligence1 notes that the market is valued at US$659.54 million (£533.83 million) and, over the period 2023–2028 is projected to reach US$3,915.73 million, registering a compound annual growth rate of 35.13%.
ADDING CONNECTIVITY
Near-field communication (NFC) technology, a short-range wireless technology supplied by semiconductor companies like NXP, makes it possible to deliver these kinds of services, creating secure patient experiences that are both beneficial and easy to use.
Perhaps best known as the wireless technology that makes contactless payments possible, NFC is readily available on most smartphones, and can be built into smart medical devices using a built-in NFC reader to automate processes.
NFC can be added to a medical device in a number of ways. If, for example, the device is already equipped with electronics and a power source, then an NFC reader frontend or NFC controller can be added to the design. Alternatively, if the medical device is purely mechanical, then passive NFC tags or connected NFC tags, which can harvest energy from the NFC field generated by the reader (e.g. a smartphone), can be used to add connectivity to the device without requiring other electronic components or a battery (Figure 1).
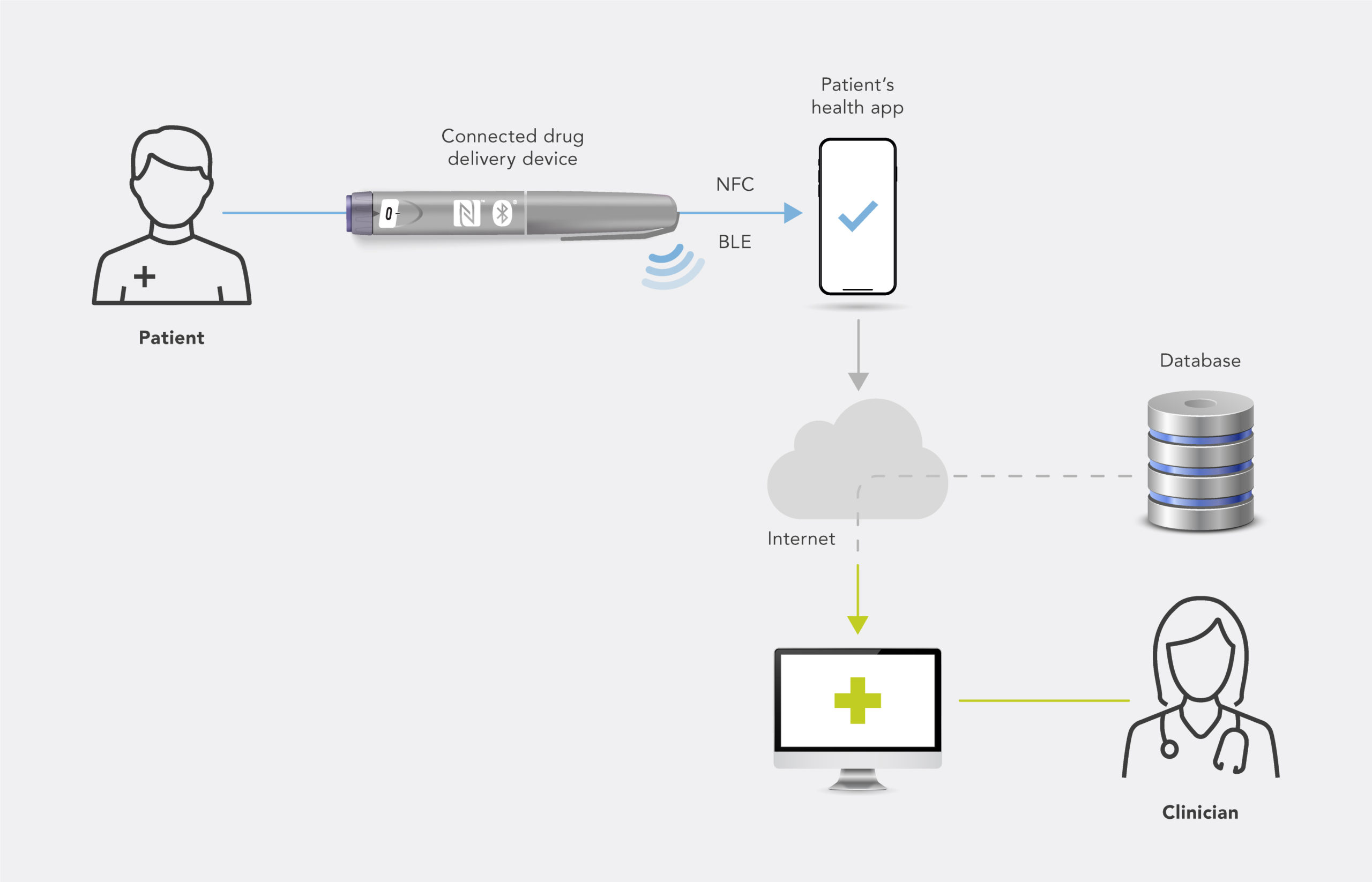
Figure 1: Example system of a connected device.
Connected devices can send data directly to a smartphone via Bluetooth, or can use one-tap NFC through patient interaction. Patients can then access a rich, interactive interface where data sets with corresponding timestamps can be stored or shared.

Figure 2: Smartphone-based patient support.
SMARTPHONE-BASED SUPPORT
The majority of patients in every age group already have an NFC-capable smartphone. Making use of this communication technology for medical devices empowers patients and lets them manage their health with a device that already feels like part of their identity (Figure 2).
With a simple tap of an NFC-enabled phone, patients have easy access to product information, can receive step-by-step application guidance (e.g. via a video), can check product authenticity or get a link into a helpful patient app to do things such as keeping a digital diary of their regime. Therapy adherence can be tracked more reliably with automated timestamps, and doses can be archived in a cloud-hosted database that records which batch of medication was administered when and to whom. This also brings obvious potential benefits for statistical analysis, since side-effect information can be captured directly in the patient app.
Depending on the use case, patients may have to install an app on their smartphone before initiating an interaction, or they may be able to use existing internet connectivity – by tapping their smartphone to the device – to launch a website that then provides digital guidance. Some data processing can take place on a mobile app, to display information in real time to the patient, or data can be sent to the cloud for more complex analysis. Patients who also use a smartwatch for heart rate or sleep monitoring may even be able to directly measure and see the effect of the drug regime on their health, reinforcing their perceived empowerment and motivating them to continue treatment.
“Sensor-based feedback can confirm that medications are handled and dosed correctly.”
SMART-DEVICE/CONSUMABLE INTEGRATION
NFC can be used to automate communication between a reusable drug delivery device and a disposable drug container, such as a cartridge or prefilled syringe. In this case, the delivery device is equipped with an NFC reader, which communicates directly with a passive NFC tag in or on the container. The NFC transaction can be used to assure the originality of the consumable at the point of use, and to check that the drug is within its expiry date. The NFC communication can also confirm the type of drug and its batch number, and it can read out delivery parameters that might require an adjustment of device settings. Moreover, the NFC reader can register dosage events, even on the consumable’s NFC tag, thereby preventing consumable reuse (Figure 3).
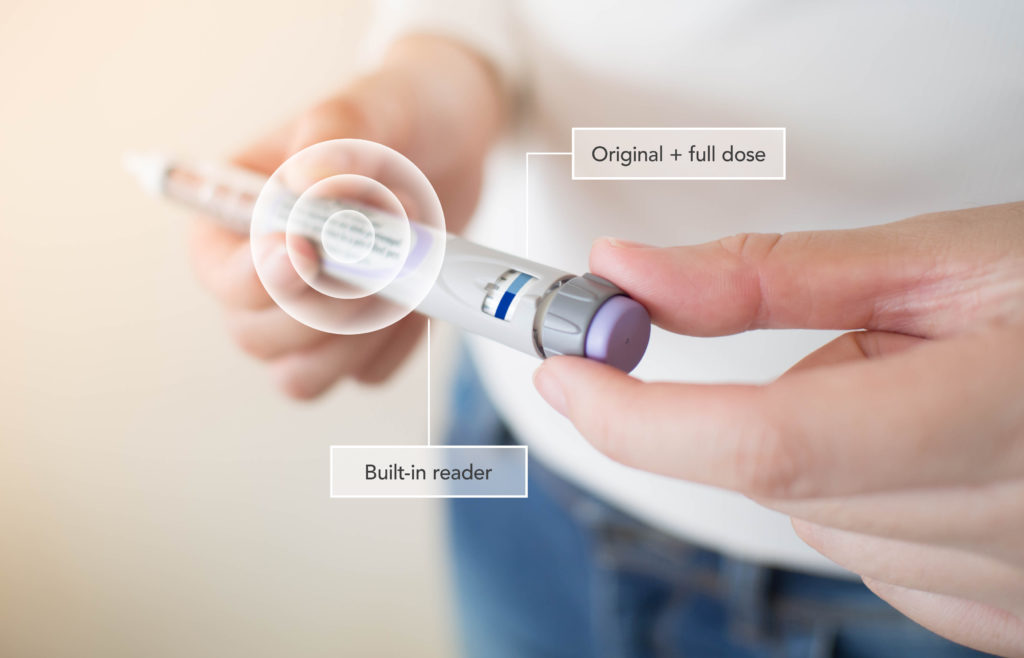
Figure 3: Communication between device and medication.
NFC tags can also be equipped with sensing capability, making it possible for the delivery device to play a greater role in supporting self-administration. Sensor-based feedback can confirm that medications are handled and dosed correctly. NFC tags that include a capacitive sensor structure can detect mechanical movement within the device or sense a change in the fill level of a drug container (e.g. full, empty or in-between states) to ensure dosages are administered fully and correctly.
CONNECTIVITY AND PATIENT ADHERENCE
The therapeutic efficacy of a drug depends not only on its pharmacology and bioavailability but also on patient compliance with the treatment regimen. However, many patients with chronic illnesses miss doses, take the wrong dose or stop treatment altogether. Reasons for poor dose adherence and compliance are often associated with symptomatic aspects of the disease, negative side effects of the drug, dose frequency, over-/under-dosing of a drug and usability of the delivery device. The result is poor health outcomes for patients.
With connected devices, patients can manage their condition while receiving immediate feedback to guide them through administration. Rather than manually recording data themselves, they can use automated features to effortlessly capture key information, such as injection dose, date and time. Also, notifications can help remind patients when their next dose is due and or send an alert when doses are missed.
Collected adherence data can also be uploaded to a cloud-hosted database through an active network connection, where more complex algorithms instruct data processing to extract patterns and inform therapy changes. It is also worth investigating how data could be further shared with existing healthcare systems, such as electronic patient records, or how information could keep patients’ clinicians informed as part of automatic uploads or downloads in the doctor’s office.
SECURITY AND PATIENT REASSURANCE
To reassure patients worried about the security of their drug delivery combination product, and to prevent the use of wrong or fake products, manufacturers should consider adding authentication and authorisation processes beyond mere identification. Unfortunately, counterfeit, adulterated, off-label and diverted products have become more and more widespread. The WHO describes counterfeits as “one of the urgent healthcare challenges of the decade”, aggravated by the globalisation of supply chains and a growing share of e-commerce.
Authentication verifies that a device or its medication is genuine, securing access to an application or its data. While identification validates serial numbers using online whitelists, NFC-based authentication performs enhanced checks based on an item’s NFC tag data and credentials, using passwords or secure cryptographic functions. Authorisation can further check attributes of each tag, such as ensuring rightful access of users to their data or delivery system.
“A mutual authentication mechanism can ensure only an authenticated reader in a device can access sensitive tag data, protecting such data from unauthorised access or malicious modification.”
NXP SMART TECHNOLOGY SOLUTIONS
NXP is using its position as a leading semiconductor company with advanced solutions for wireless connectivity, sensors and microcontrollers to support connected devices for personal and clinical use that deliver excellent quality of care and security.
NXP offers a range of solutions for NFC and, to address concerns over the security of patient data and the possible presence of counterfeit products in the supply chain, offers multiple functions that safeguard access to user data and check the authenticity of devices and drug consumables.
Secure NFC Tags
NXP’s NFC security tags combine standard based AES-128 cryptography for tag and message authentication with enhanced functionality, such as status detection. The company offers products with independently certified security algorithms backed by Common Criteria security certification. An optional scheme for mutual authentication ensures both reader and tag share the same secret or key, so the tag releases data only to an authenticated reader, thereby protecting it against unauthorised access or malicious modification attempts.
NXP NFC tags include the popular NTAG® DNA (ISO 14443/Type 2 or 4 Tag) and ICODE® DNA (ISO 15693/Type 5 Tag), which support a range of operating distances and form-factor dimensions. NXP’s certified NTAG 22x and 42x DNA tags come with Advanced Encryption Standard (AES)-128 cryptography and web-based secure, unique NFC (SUN) message authentication for dynamic, secured NFC messages on every NFC phone readout to prevent mass copying. For an automated offline authentication in a device, a mutual authentication mechanism can ensure only an authenticated reader in a device can access sensitive tag data, protecting such data from unauthorised access or malicious modification.
NXP’s latest NTAG 22x DNA Status Detect (ISO1443/Type 2 Tag) combines security with innovative status sensing using capacitance. When interrogated, the tags can capture a capacitance variation, resulting from a change in condition, and these variations can be interpreted with a software application. This feature can be used to measure the fill level of liquid drug containers or to capture mechanical movements in medical devices.
Connected NFC Tags
Connected NFC tags are devices that, in addition to their regular contactless communication functions, can harvest electrical energy and communicate through a wired interface. Connected NFC tags can provide power to an external sensor or a microcontroller, or to LEDs or switches. In medical devices, they can help measure temperature to make sure an injectable drug is within the expected temperature window, and they can measure pressure to help control an inhalation device or measure if a mechanical function is complete. Results can be stored locally but can also be made available for download to the user’s NFC smartphone.
The NTAG 5 (ISO 15693/Type 5 Tag) and NTAG I2C Plus (ISO14443/Type 2 Tag) are versatile connected NFC tag solutions for use in or on a system with an electrical system interface to connect to, for example, a microprocessor or sensor in a device via an I²C bus, and support memory data protection with segmented access rights management.
Single-Chip NFC Microcontroller
NXP’s single-chip NFC microcontroller PN7642, released in March 2023, offers the highest integration level and the smallest footprint for adding NFC reader functionality, processing and security, even in small drug delivery devices. The PN7642 embeds a high-performance NFC frontend, delivering up to 2W output power and optimised for battery-powered application, an Arm Cortex M33-based microcontroller with 180 kB customisable flash memory and a full security toolbox supporting cryptographic authentication with internal key storage. The PN7642 is SESIP Level 2 security certified to resist software attacks (Figure 4). For devices requiring protection against more sophisticated hardware attacks, NXP offers Secure Elements (SEs) and Secure Access Modules (SAMs) for enhanced key protection.
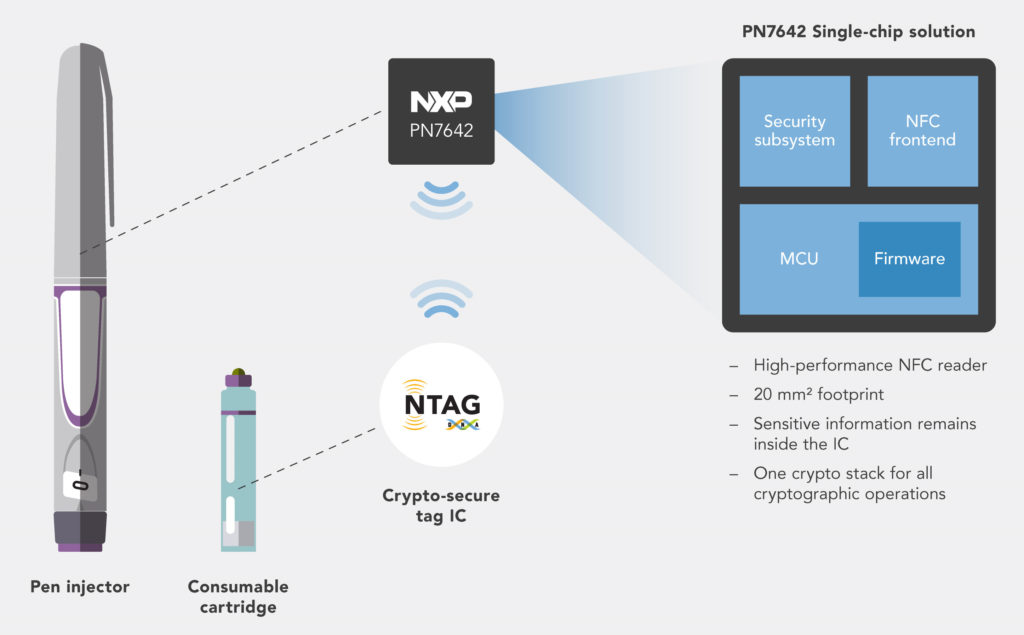
Figure 4: Possible device-consumable system enabled by NFC.
LOOKING AHEAD
Connectivity will continue to play an important role in maximising the quality of healthcare for patients, especially those with chronic conditions. NXP is building on its reputation as an innovation leader to deliver high-quality, reliable solutions for connected drug delivery devices. The company’s extensive partner network, supported by a comprehensive ecosystem of development resources, helps companies develop their next generation of drug delivery solutions.
REFERENCES
- Mordor Intelligence, “Connected Drug Delivery Devices Market Size & Share Analysis – Growth Trends & Forecasts (2023 – 2028)”. Research Report, Mordor Intelligence, accessed May 2023.

