Citation: Reynolds J, “Enhancing the Patient Experience for Self-Injection Systems”. ONdrugDelivery Magazine, Issue 105 (Feb 2020), pp 36-40.
The complexity of injectable treatment regimens for chronic diseases means onboarding aids can be integral to disease management. Joe Reynolds discusses the role of demonstration devices in improving the patient experience and adherence.
Diagnoses of chronic illnesses such as rheumatoid arthritis (RA), Crohn’s disease and osteoporosis are expected to skyrocket in the coming decade, resulting in growing use of patient-centric drug delivery devices – including autoinjectors, prefilled syringes with safety features, and on-body and respiratory devices.
“Retention and recall worsen over time without practice and repetition.”
Because of the complexity of injectable treatment regimens, onboarding aids – such as reusable demonstration devices – can be integral to the disease management process, from improving compliance to enhancing the patient experience and easing their emotional responses to disease-related changes in their lives. Yet, nearly half (49%) of patients do not receive in-office training when prescribed self-injection medication.1
When patients are properly trained and given the tools for success at the time of their therapy prescription, they can become more adherent – receiving the full benefit of their therapy, leading to longer, healthier lives. What is striking is the lack of training that healthcare professionals (HCPs) provide. Studies reveal that, after prescription, 84% of patients incorrectly use their autoinjector at home.2
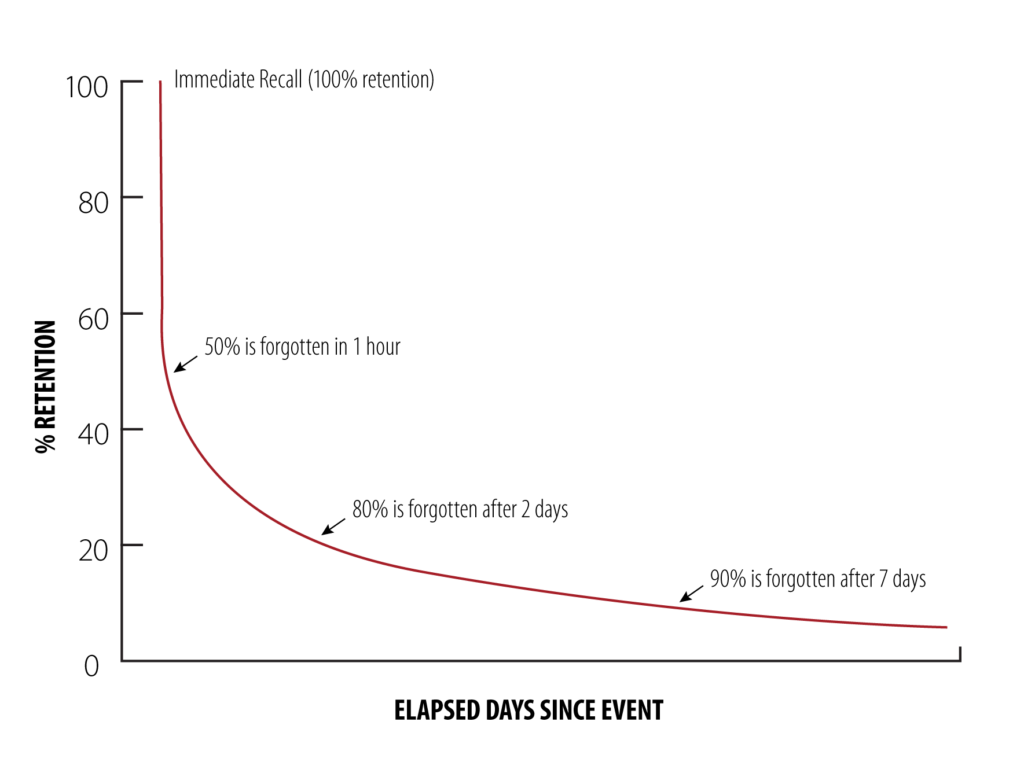
Figure 1: The forgetting curve posits that, without practice and repetition, retention and recall degrade over time.
Another phenomenon working against patient adherence is the forgetting curve theory that suggests retention and recall worsen over time without practice and repetition. This theory postulates that 50% of the information HCPs give to their patients when prescribing a self-injection is forgotten within one hour, 80% is forgotten in two days and 90% is forgotten in a week (Figure 1).3
As biologics become more advanced and require less-frequent dosing, the longer down-time between injections may allow for non-adherent patient behaviours to arise. This is especially troublesome for patients who are tasked with self-injecting without the support of an HCP — or even a demonstration device — week after week throughout their therapy.
Demonstration devices closely replicate actual prefilled syringes and autoinjectors but without the needle or medicament. This allows patients to learn how to use a specific device and potentially improve familiarity, increase confidence and build adherent behaviours with the true drug delivery device and medication. This intermediate step in the self-injection journey may not only increase health outcomes for the patient but also can reap benefits for HCPs, pharma companies and payers in the form of decreasing healthcare costs.
With so much to gain, both therapeutically and financially, when patients are properly trained on the use of self-injectors, it is incumbent on the healthcare community to determine how best to ensure this adherence.
DEMONSTRATION DEVICES IN CLINICAL TRIALS
Successfully developing and launching combination products is an intricate process. As compounds mature through early-stage clinical trials, profiles and formulations are analysed to determine the optimal route of administration and presentation for product candidates. When it comes to biopharmaceuticals, this commonly includes selection of the drug delivery systems that will be used to distribute and administer the medications.
“Testing drug delivery devices with the end users in the early stages
can help facilitate greater success downstream.”
Manufacturers often conduct market research and human factors studies when designing and developing delivery systems to select a device that satisfies the needs of patients, HCPs and other stakeholders.
During this process, outputs from these workstreams provide valuable insight into patient needs – from prescription and onboarding through the entirety of their use of the therapy. The insights gathered and examined during this process should be leveraged to help define and prioritise requirements for demonstrators and other educational resources for patients as they embark on their new therapy.
When this process occurs in the early stages of clinical trials, there is opportunity for pharma companies to use demonstration devices during human factors studies to determine the best route of administration to aid patient comfort and, more importantly, long-term adherence. Testing drug delivery devices with end users in the early stages can help facilitate greater success downstream.
Demonstration devices may be used in human factors studies during early-phase clinical trials to create best-in-class user experiences by:
- Better understanding the needs and expectations of patients and HCPs to create robust and impactful training and onboarding solutions that support patients from prescription of their new self-injectable through their at-home treatment and beyond.
- Educating users and helping promote the efficient progression of programmes and collection of quality data.
For demonstration devices to be used effectively during these early-phase clinical trials, best practices should be established to ensure their use brings the most value to the study and to patients, HCPs,pharma companies and payers long term. As such, it is important to create a framework that dictates the use of demonstration devices in these, to:
- Train sponsor personnel and site investigators on the use of devices to ensure they are correctly administering agents and training patients, where applicable. Ensure sponsor personnel understand the difference between various self-administration devices and the benefits and potential drawbacks of each
- Qualify study participants by allowing them to experience how various devices operate and ensuring they can self inject as directed. It is important to ensure subjects begin on an even playing field in terms of their knowledge of device usage
- Allow trial subjects to build their confidence in self-administration and to gain hands-on experience in using a drug delivery device
- Provide ongoing training to subjects and study personnel in order to mitigate learning erosion – as per the forgetting curve theory – and improve the recall and application of skills, particularly for at-home dosing
- Use resettable demonstrators rather than real drug delivery devices during the clinical and device development process to minimise waste
- Improve user safety by reinforcing proper injection behaviours through resettable devices.

Figure 2: Patient using the Noble
demonstrator of the BD UltraSafe
to practise a self-injection.
A great deal of useful information can be gleaned if these recommendations are followed, including patient and/or HCP preference for one drug delivery device over another, patient knowledge gained from using a training device, understanding how hands-on experiences using a demonstration device can successfully change the way patients self-inject, and how well training can help mitigate learning erosion. These insights can enlighten the pharma industry in near limitless applications (Figure 2).
NOBLE AND BD COLLABORATE ON BD ULTRASAFE™
Although BD has not yet used demonstrators in clinical trials, one example that supports this use is Noble and BD’s partnership for the BD UltraSafe™ needle-guard product family. Noble and BD established a collaborative development framework in an effort to:
- Help pharma companies increase touchpoints through access to and use of demonstrators in a variety of settings by patients, caregivers, HCPs, pharmacies and product-specific channels
- Support patient adherence and confidence with self-injection therapies across the board.
The two companies understood the importance of providing demonstration devices to pharma companies as a method to enhance patient education programmes for the rising number of at-home self-injectable therapies. They also understood that, with this rise, comes a growing number of patients who are expected to administer their therapies without the support of an HCP – which has the potential to lead to decreased patient adherence.
Noble and BD’s collaboration for the BD UltraSafe includes a customisable demonstrator platform for manufacturers developing and launching drug delivery systems. BD UltraSafe is increasingly used to deliver self-injections across a breadth of therapies for diagnoses ranging from RA, osteoporosis and psoriasis to asthma and migraines, among others.
ABOUT THE DEMONSTRATOR
The Noble demonstrator of the BD UltraSafe system is designed for repeated patient use. It is true in form and function to the actual BD UltraSafe system – producing a realistic injection experience – and comes with various needle options.
To facilitate at-home patient engagement with the product, the demonstration device comes with its own instructions for use that teach patients not only the proper injection steps but also how to reset the device for repeated use. Additional videos and how-to material can also be included to bolster patient engagement with the product further and thus support therapy adherence and confident self-injection.
“Demonstration devices are vital components of the patient self- injection experience.”
The Noble demonstrator platform for the BD UltraSafe system can be slightly modified for brand specifications and other nuances, as required, but otherwise facilitates provision of demonstrators that are ready for market. The benefits of Noble’s platform devices for pharma companies interested in deploying a demonstration device with their BD UltraSafe passive needle guard include:
- Speed to market: demonstration devices are off-the-shelf ready for market
- Accurate simulation: BD device design specifications form an integral part of the demonstration device creation
- Proprietary design: patented technologies provide repeatable and reliable training experiences
- Quality: demonstration devices are tested against Noble’s stringent quality standard
- Low cost of entry: there is no tooling expense.
These products are tested and validated through tooling and process validation, design verification testing, including lifecycle testing, and product functionality testing.
When compared with non-platform demonstration devices, the Noble demonstrators of the BD UltraSafe system differentiate themselves by being market ready, making it even more important for pharma companies to know which type of delivery device will be used for their drug very early in the development process. Once that is understood, a demonstration device can be deployed to market concurrently with the drug and its delivery system, supporting the launch and solidifying the demonstration device – in the eyes of patients – as an imperative tool for treatment success (Figure 3).

Figure 3: The Noble demonstrator of the BD UltraSafe product line.
CONCLUSION
Demonstration devices are vital components of the patient self-injection experience. But determining the correct device requires specialised planning, co-ordination and expertise. When properly implemented, along with other patient support services, demonstrators and injection training can help improve patient adherence.
When patients stay on their therapies longer and more consistently, it is a win-win for all involved, including HCPs, pharma companies and payers. Achieving these benefits requires deliberate planning early in the product lifecycle, starting as early as the clinical trials. Doing so increases the contributions of demonstration devices throughout the product lifecycle, from use in clinical trials through to the patient use feedback loop.
To read more about BD and Noble’s collaboration on the BD UltraSafe™ passive needle guard product family and the importance of utilising demonstration devices in clinical trials, please visit: bit.ly/EnhancingPatientExperience.
BD and BD UltraSafe are property of Becton, Dickinson and Company. Noble’s demonstrators are multi-use.
BD UltraSafe™ devices are single-use medical devices.
REFERENCES
- Lang V, Nalan D, “Combination Product Patient Training: How Are Patients Trained and Who Conducts the Training?”. Proc 2018 Design of Medical Devices Conference (Am Soc Mechanical Engineers), April 9-12, 2018, Minneapolis, MN, US, DMD2018-6956.
- Potera C, “Misuse of Autoinjectors and Inhalers”. Am J Nursing, Vol 115(3), p17.
- Kohn A, “Brain Science: The Forgetting Curve –the Dirty Secret of Corporate Training”. Learning Solutions, March 13, 2014.

