To Issue 133
Citation: Metzmann F, Muenzer C, “Excellence Through Simplicity: PiccoJect Autoinjector Platform”. ONdrugDelivery, Issue 133 (May 2022), pp 63–68.
Fred Metzmann and Chris Muenzer discuss the simplicity of the PiccoJect™ autoinjector platform and the company’s commitment to sustainability.
Effective self-management of chronic diseases requires healthcare solutions that are easy and convenient for patients. This is one of the key drivers for Haselmeier’s patient-focused development of innovative drug delivery solutions.
The simplicity of PiccoJect, combined with the company’s sustainability philosophy, is reflected in the entire supply chain to reduce waste and minimise environmental impacts. In addition to investing in green electricity and the use of sustainable feedstocks, Haselmeier focuses on the development of regional supply chains for the US and European markets. “Excellence through simplicity” sums up the company’s patient-centred and sustainable philosophy.
“Injection systems for self-administration have evolved into a pivotal tool for the effective treatment of chronic diseases.”
CHRONIC DISEASES ARE ON THE RISE
The number of chronic diseases and conditions is increasing worldwide. Changing social behaviours and ageing populations are the main causes of the steady spread of these common and costly diseases. Emerging economies, with their rapid population growth, are the most affected.
The WHO estimates that non-communicable chronic diseases cause some 41 million deaths per year1 – equivalent to 71% of all deaths globally.2 It also predicts that the prevalence of chronic diseases will reach 49% by the year 2030.3
Rapid progress in the development of medicines, combined with more effective therapies, can help improve and prolong the lives of patients suffering from such diseases.
In 2021, a total of 50 new drugs were approved by the US FDA – 36 as new molecular entities (NMEs) under NDAs and 14 as new therapeutic biological products under BLAs excluding FDA approvals for generics and biosimilars.4 The terms biologics and biosimilars refer to various substances – including antibodies, therapeutic proteins and peptides – that have a biological origin.4-7
Biologics will change medical practice gradually due to their distinct advantages in efficacy and selectivity, and their application will steadily expand. To date, the most considerable growth has occurred in the therapeutic areas of cancer and cancer-related diseases, rare diseases, neurological disorders and autoimmune diseases.7 However, the specific physicochemical properties of biologics mean that delivering them as active agents into the body can be challenging. As a result, innovations in drug development today are not only limited to substances and therapies but also include the patient-centred development of delivery devices. These systems play an increasingly important role in making administration easier, faster and more efficient.
SELF-ADMINISTRATION A KEY ELEMENT IN THE EFFECTIVE TREATMENT OF CHRONIC DISEASES
Self-administration has become increasingly important in this context. Set to surpass oral and other routes of administration, parenteral drugs are expected to see their market share grow from approximately US$600 billion (£478 billion) in 2019 to approximately $1,200 billion by 2026.8,9,10 This global growth is driven mainly by the growing importance of preventive medicine, the ageing population and the general shift towards home care.7,8,9
The vast majority of biologics are currently administered intravenously and subcutaneously. This applies to both biologics that are already approved and marketed and to biologics in development. However, subcutaneous self-administration is increasing in prevalence and represents a valuable alternative to intravenous administration. With a 41% share, subcutaneous bolus injection is already the preferred route of administration for biologics in the development pipeline.6,7,14,15
This growth is mainly due to the safety and effectiveness of subcutaneous dosing, which both patients and healthcare professionals greatly appreciate. After a short training session, patients and/or healthcare professionals can administer the drug very quickly at home. This leads to an improved quality of life, a reduction in the time spent travelling to the healthcare facility and, consequently, a reduction in costs and environmental impact. Again, this aligns strongly with Haselmeier’s approach of “excellence through simplicity”.
Injection systems for self-administration have evolved into a pivotal tool for the effective treatment of chronic diseases. The growing demand for such systems is driven by factors such as the convenience patients enjoy when safe and easy self-administration at home saves them the time and effort involved in trips to healthcare facilities. At the same time, autoinjectors are straightforward and safe to use by patients or their caregivers, reducing the burden on more highly skilled healthcare professionals.5,16 Flexible care at home is not only convenient for patients but also has a positive impact on sustainability. Far fewer ambulance transports or trips to the hospital or clinic are required. In addition, the number of hospitalisations is significantly reduced.17,18,19
“The company’s approach to connectivity aims to minimise any impact on the injection process, reduce patient training and eliminate the need for a patient app.”
ADDRESSING THE ENTIRE VALUE CHAIN
In view of the long-term nature of chronic diseases, a favourable patient experience is paramount. However, continuous improvements in ease of administration, pain reduction, compliance and adherence are just part of the picture. Other key aspects that call for attention include the quality of the entire value chain in the drug delivery process.
With healthcare and pharmaceutical experts on the Haselmeier team, multiple interviews were conducted with customers and subject matter experts in drug delivery devices and combination products. These interviews addressed the drug delivery value chain from end to end – from the conceptual idea of the drug delivery solution, through its design and manufacture, to the packaged combination product, including its supply and use. The team also reviewed feedback and enquiries from clinicians, plus discussions with current users of existing autoinjectors. The findings identified several unmet needs that inspired the team to develop a new generation of autoinjectors that seamlessly combine functionality, convenience, user-friendliness and sustainability.
The outcome of this development is the PiccoJect autoinjector platform. Its ease of use, inherent safety and sustainability characteristics set this platform apart from existing offerings in the marketplace. It includes two variants of the highly compact PiccoJect autoinjector, supported by a rich set of services (including customisation, pre- and final assembly and packaging) along the entire value chain.

Figure 1: The PiccoJect autoinjector is made up of only eight parts.
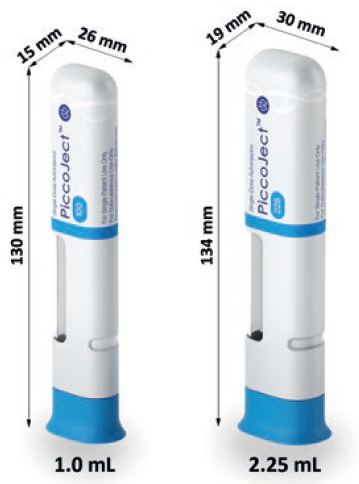
Figure 2: The dimensions of the 1.0 mL and 2.25 mL PiccoJect autoinjector.
SIMPLICITY IS KEY TO EFFICIENCY
“Excellence through simplicity” sums up the key features of the PiccoJect autoinjector design. It all starts with an extremely low part count: the PiccoJect autoinjector is made up of only eight parts (Figure 1). Apart from the syringe, the highly integrated delivery mechanism consists of five injection-moulded plastic parts, two springs and one metal component.
The delivery mechanism of the PiccoJect autoinjector accommodates any standard 1 mL long and 2.25 mL prefilled glass or plastic syringe with a small round or cut flange (Figure 2). The same mechanism is available with two different cross-sections, tailored to the applicable syringe size to provide two discrete and user-friendly device form factors.
DELIVERING A BETTER USER EXPERIENCE
To help ensure adherence, the PiccoJect autoinjector is designed from the ground up for ease of use (Figure 3). It features a large wrap-around drug window for visual inspection of the drug prior to use, visual and audible feedback on the device status, and integrated needle safety. These features, plus its small size, slightly flattened (rather than circular) cross-section and intuitive feedback make this device particularly straightforward to handle.
PiccoJect is a compact, customisable and intuitive two-step autoinjector for safe and convenient self-administration, designed for subcutaneous delivery of drug products. To keep patient discomfort to a minimum, the needle guard provides a relatively large contact area that reduces pressure on the skin. Audible clicks at the start and end of each injection, as well as visual feedback, help ensure the user holds the device in place until the full dose has been injected. A dedicated status indicator provides easy-to-understand binary information about the usage status of the autoinjector. For example, upon completion of the injection procedure, the coloured status indicator notifies the user that the syringe is depleted and the device has been used.
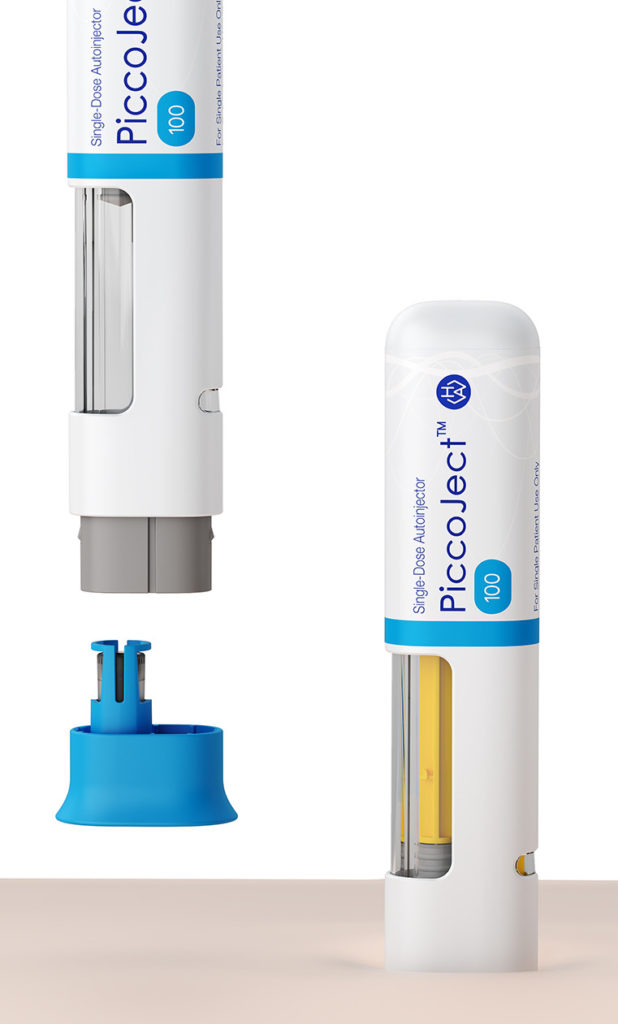
Figure 3: Detailed view of PiccoJect features. Left – the cap must be removed before use. Right – audible clicks at the start and end of each injection, as well as visual feedback, help ensure the user holds the device in place until the full dose has been injected.
INTELLECTURAL PROPERTY POSITIONING
Part of Haselmeier’s intellectual property (IP) strategy is to constantly maximise and expand patent protection for PiccoJect technology through continuous innovation and new applications. Haselmeier has already filed several patents for the PiccoJect autoinjector, covering various innovative technologies, safety features and digitisation (Table 1).
| PiccoJect – 100 | PiccoJect – 225 | |
| Part count | 5 plastic components, 2 springs, 1 metal component |
|
| Primary container |
1 mL long glass or plastic syringe |
2.25 mL glass or plastic syringe |
| Syringe flange | Small round or cut flange |
Small round or cut flange |
| Fill volume (how to adapt the fill volumes) |
0.2–1 mL | 0.6–2 mL |
| Injection time* | <10 s | <15 s |
| Viscosity** | Up to 20 cP | |
| Needle insertion depth |
6 mm (nominal); customisation possible | |
| User feedback | Audible click at start and end of dose; visual feedback in dose window and dedicated status indicator |
|
| Needle safety | Automatic needle shielding with needle hidden before, during and after use |
|
| Needle type and gauge |
27G and 29G, normal wall through special thin wall |
|
| Weight | 26 g (without syringe) |
34 g (without syringe) |
| Dimensions | H = 130 (cap on), W = 26, D = 15 mm |
H = 134 (cap on), W = 30, D = 19 mm |
*, ** Injection time and viscosity capability are dependent on needle diameter and fill volume.
Table 1: The specifications of PiccoJect.
“The underlying design concept of the PiccoJect autoinjector was driven by sustainability requirements.”
CUSTOMISATION OPTIONS
Thanks to its versatile design, the PiccoJect autoinjector can adapt easily to a range of customer requirements. Standard customisation options include part colour, the size of the drug window and spring force. Agility is key: these customisation options are available without any detrimental impact on development timelines.
CONNECTIVITY FOR DATA-DRIVEN INSIGHT
Currently, Haselmeier is developing connectivity options that will enable the PiccoJect platform to integrate seamlessly with existing digital ecosystems, allowing injection-related data to be collected automatically at the point of care (Figure 4). The company draws on experience gained through its D-Flex Ecosystem and D-Flex Logbook technologies. The idea is to equip the PiccoJect autoinjector with a smart add-on that collects data and transmits it to a private cloud for clinical evaluation. Again, the guiding principle is excellence through simplicity. The company’s approach to connectivity aims to minimise any impact on the injection process, reduce patient training and eliminate the need for a patient app.

Figure 4: Connectivity options allow injection-related data to be collected automatically at the point of care.
Smart medical devices that drive a steady increase in self-care, personalisation of treatments, predictability of outcomes or proactive intervention will help elevate the quality of care to a new level. Connected drug delivery systems in digital healthcare workflows are starting to prove their worth. For example, a clinical trial conducted by a major player in healthcare monitored the adherence patterns of 75 diabetes patients with the help of a connected drug delivery device.20 The trial results underscored the need to gain a better understanding of patient behaviour and adherence to the prescribed therapy.
COMMITTED TO SUSTAINABILITY
At Haselmeier, sustainability is embedded in daily business and in the foundations of the corporate strategy (Figure 5).21 The company’s sustainability objectives address the rights and needs of people, profitability and the necessities of protecting our planet against severe short- and long-term impacts.
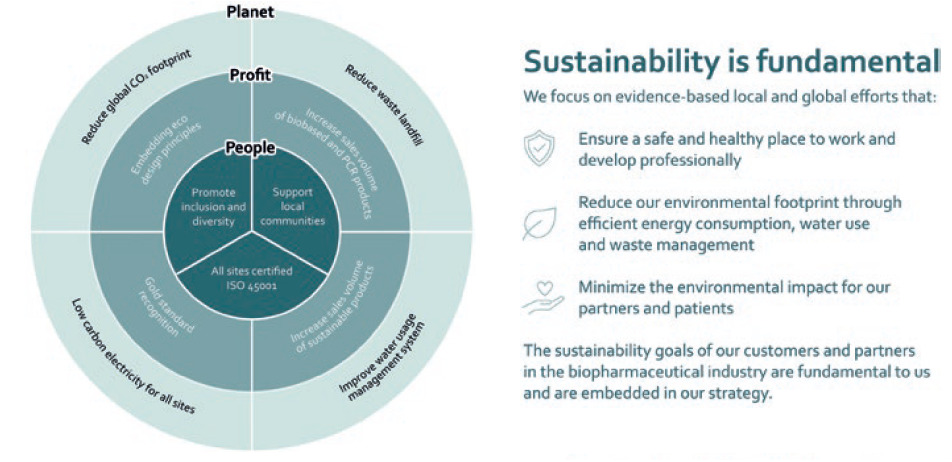
Figure 5: The sustainability goals of Haselmeier, a medmix brand.
In line with the global sustainability strategy pursued by medmix, Haselmeier is proactively implementing steps aimed at reducing its global carbon footprint, reducing waste landfills, improving its water usage management systems and implementing low-carbon electricity throughout its sites. Haselmeier development and design initiatives embrace sustainability during the entire lifecycle of its products. The sustainability goals of customers and partners in the biopharmaceutical industry are equally important. The Haselmeier sustainability objectives are in line with the emission reduction goals of the pharmaceutical industry. Currently, this global industry is driving major initiatives for reducing its carbon emissions.22 It is Haselmeier’s corporate strategy to support the industry in achieving its ambitious goal of greater eco-friendliness.23 Haselmeier proactively addresses such issues and is willing to collaborate with customers to jointly develop and implement appropriate solutions for reducing emissions and driving sustainability. It is committed to implementing specific measures along the entire value chain from conceptual design, manufacturing and packaging solutions, through to supply chain topics.
The sustainability efforts have not gone unnoticed. The Haselmeier s.r.o manufacturing site in the Czech Republic has received the prestigious silver rating from EcoVadis, one of the world’s most trusted business sustainability rating entities. It achieved a score of 63/100 and performed particularly strongly within the environment, labour and human rights sectors. With the same sustainability practices incorporated throughout its sites in Europe, the company is on the right track.
The underlying design concept of the PiccoJect autoinjector was driven by sustainability requirements. Its parts were optimised to allow for the use of materials with minimal environmental impact – such as plastics based on attributed biocircular key raw materials via a mass balance approach. In line with the medmix approach to keeping shipping routes short, manufacturing and distribution facilities are located in the geographies where customers and partners are based. The PiccoJect autoinjector will be manufactured at Haselmeier sites that rely on low-carbon electricity.
HASELMEIER ECO-DESIGN PRINCIPLES
Disposable drug delivery devices still offer advantages in terms of ease of use and reimbursement over reusable products, especially for less-frequent injections. In addition, contamination and hygiene issues impose limitations on the circularity of healthcare products. Consequently, Haselmeier believes that the pharmaceutical industry and its customers will continue to request disposable products for some time to come. To offset this impact, Haselmeier’s eco-design principles aim to reduce the environmental footprint of these products by:
- Optimising component wall thicknesses to minimise excess materials
- Avoiding the use of materials with high carbon intensities, such as polyamide 6
- Selecting eco-friendly materials where the supplier provides sustainable feedstock options
- Minimising the use of tray handling for individual components during assembly
- Manufacturing trays and cartons from post-consumer materials.
Haselmeier is always open to new ideas and happy to support clients from clinical studies to commercial launch of their combination products.
DISCLAIMER
The products shown in this article are under development and some of them may not yet have been approved for sale under applicable medical devices regulation. The content provides general informationabout these products, their field of application and intended use, and their function, performance and specification are subject to customer specific development and may deviate from those shown herein. All information contained herein is directed at medical device manufacturers and pharmaceutical companies. This information shall not constitute a promotion for use in a way which conflicts with any applicable medical device regulations – nor is it directed at patients or intended to replace professional medical advice.
REFERENCES
- “Noncommunicable diseases”. Fact Sheet, WHO, Apr 13, 2021.
- “Noncommunicable diseases”. Health Topics, WHO, Feb 3, 2022.
- “Percentage of the Population With Chronic Diseases, 1995-2030”. The Silver Book, May 2009.
- “Advancing Health Through Innovation: New Drug Therapy Approvals 2020”. US FDA, Jan 2021.
- De la Torre BG, Albericio F, “The pharmaceutical industry in 2018. An analysis of FDA drug approvals from the perspective of molecules”. Molecules, 2019, Vol 24(4), p 809.
- Ganesh AN et al, “Patient-centric design for peptide delivery: Trends in routes of administration and advancement in drug delivery technologies”. Med Drug Discov, Mar 2021, Article 100079.
- Jonaitis L et al, “Intravenous versus subcutaneous delivery of biotherapeutics in IBD: an expert’s and patient’s perspective”. BMC Proc 15, 2021, Article 25.
- “Industry Roundtable: Parenteral Drug Manufacturing”. DCAT Value Chain Insights, Feb 10, 2021.
- “World Preview 2020, Outlook to 2026”. EvaluatePharma, Jul 2020.
- “The Prospects for Biosimilars of Orphan Drugs in Europe – Current Landscape and Challenges Ahead”. IQVIA, Institute Report, Jul 21, 2020.
- “Global Medicines Use in 2020 – Outlook and Implications”. IMS Institute for Healthcare Informatics, Nov 2016.
- “The Pharma Pulse: Small Molecules and Biologics”. DCAT Value Chain Insights, May 26, 2021.
- “The Congruence Between Small Molecule Generic Medicine and Biosimilar Medicine Business Models”. Am Pharm Rev, Featured Articles, Oct 15, 2017.
- De Cock E et al, “A time and motion study of subcutaneous versus intravenous Trastuzumab in patients with HER2-positive early breast cancer”. Cancer Med, 2016, Vol 5(3), pp 389–387.
- Bittner B, Richter W, Schmidt J, “Subcutaneous Administration of Biotherapeutics; an overview of current challenges and opportunities”. BioDrugs, 2018, Vol 32(5), pp 425–440.
- “Injectables: The New Oral?”. Contract Pharma, May 4, 2016.
- “Health care’s response to climate change: a carbon footprint assessment of the NHS in England”. Lancet Planet Health, 2021, Vol 5(2), pp e84–e92.
- Waller C et al, “Intravenous and subcutaneous formulations of trastuzumab Biosimilars implication for clinical practice”. Br J Cancer, 2021, Vol 124(8), pp 1346–1352.
- De Cock E et al, “Time savings with Rituximab subcutaneous injection versus Rituximab intravenous infusion: A time and motion study in eight countries”. PLoS One, 2016, Vol 11(6), e0157957.
- “Insulin Dosing Practices in Persons With Diabetes on Multiple Daily Injections”. ClinicalTrials.gov, Aug 14, 2018.
- “Sustainability Review”. Annual Report, medmix, 2021.
- “Big Pharma emits more greenhouse gases than the automotive industry”. Brighter World, May 28, 2019.
- “Turning Pharma Green: an Eco-Wish List for the Industry”. Pharma Technology Focus, 2020.
Previous article
Technology Showcase: CCBio’s Felice DoseNext article
A CHECKLIST FOR AUTOINJECTOR DESIGN
