To Issue 154
Citation: Thurston G, Jordan L, “Exploring the Performance of Low-GWP Propellants”. ONdrugDelivery, Issue 154 (Nov 2023), pp 37–40.
Grant Thurston and Lynn Jordan discusses the performance of pressurised metered dose inhaler propellants with a low global warming potential.
In 1987, the landmark multilateral Montreal Protocol was finalised, initiating the phasing out of chlorofluorocarbon (CFC) refrigerants and propellants to limit environmental damage. The Kigali Amendment to the Montreal Protocol was ratified by the US in 2022 and signals additional efforts to reduce the environmental damage caused by fluorinated refrigerant, including hydrofluorocarbon (HFC) propellants.1
To reduce the impact of climate change, the HFC propellants used in pressurised metered dose inhalers (pMDIs) are being phased out and devices are being reformulated with new low global warming potential (GWP) propellants. The race to reformulate existing pMDIs to use low-GWP propellants is underway. To achieve these reformulations by the time Kigali comes into force in 2033, the industry needs new tools to accelerate development.2
“The propellant is a major component of any pMDI formulation and any change to it could significantly influence overall performance.”
A pMDI is a combination drug product that has a complex relationship between the device, formulation and human actuation. The propellant is a major component of any pMDI formulation and any change to it could significantly influence overall performance. Current laboratory testing tools, such as aerodynamic particle size distribution (APSD), require considerable time investments and, because of the time requirements, do not facilitate accelerated development. New technologies are needed to rapidly screen the drug/device formulations to select high-potential candidates for advancement. Regional deposition under human-realistic conditions can provide a rapid assessment of equivalence between test and reference products.
A NEW PARADIGM FOR DEPOSITION
In vitro inhaled drug analysis (INVIDA) services provide a complete rapid screening and bioequivalence tool, using human-realistic breathing profiles and respiratory tract models to determine regional deposition. INVIDA provides the speed to determine the knowledge needed to support traditional testing methods. The INVIDA services illustrated in Figure 1 show that INVIDA is a realistic model of human anatomy. The standard induction port for cascade impaction has a 90° turn, whereas the INVIDA mouth-throat model is human-idealised, more closely resembling human anatomy.
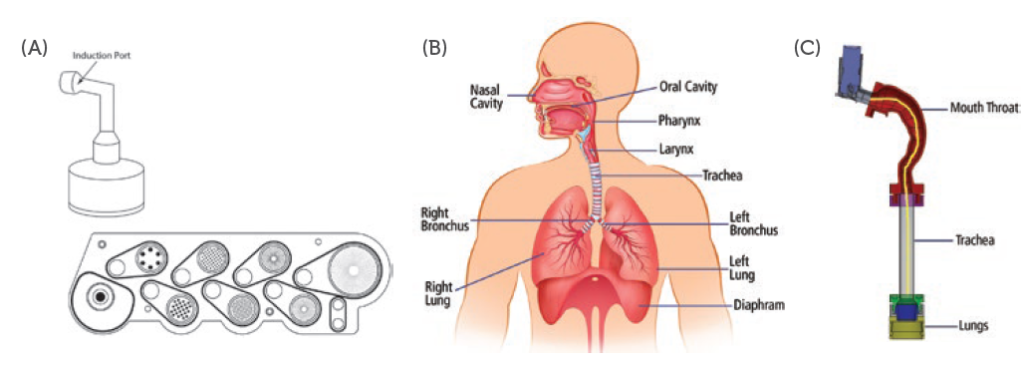
Figure 1: Visual comparison of (A) cascade impaction, (B) human anatomy and (C) INVIDA Services.
Table 1 summarises the factors associated with APSD and INVIDA services. INVIDA services enable fully customisable patient-specific breathing profiles that can represent normal breathing profiles for paediatric and adult patients, as well as mild, moderate and severe disease states of breathing. Traditional methods for APSD use a fixed flow rate for testing, whereas INVIDA can simulate realistic inhalation, hold and exhalation. Analysis by INVIDA requires approximately 25 minutes per sample for sample preparation and collection, whereas APSD will require a minimum of two hours for sample preparation and collection. Depending on the equipment, APSD can require extraction of drug from 10 components per sample run, whereas INVIDA only requires extraction from four components.
| Experimental Factor | APSD | INVIDA |
| Flow Rate | Constant | Human-Realistic (Breathing Simulator) |
| Geometry | NGI with 90° Throat Model | Human-Idealised |
| Analyst Time per Sample | 2 Hours | 25 Minutes |
| Prep / Cleaning | 10 Components | 4 Components |
| Data Output | Size Distribution and Fine Particle Mass (>5 μm) | Mouth-Throat, Trachea, and Lung Deposition (mcg) |
Table 1: Comparison of experimental factors associated with APSD and INVIDA services.
Traditional deposition studies do not measure deposition directly, but rather study particle size, which is thought to be indicative of human deposition. The INVIDA system consists of three extractable regions: idealised mouth-throat models (Virginia Commonwealth University, VA, US), trachea and lungs, yielding direct recovery of API (mcg). The speed of analysis makes INVIDA services an ideal development tool and can be used early and often to reduce dependence on costly and time-consuming APSD testing.
CASE STUDY
Proveris Laboratories evaluated a combined human-realistic deposition/APSD workflow that reduces development times while maintaining performance insights and regulatory compliance. To demonstrate the use of human-realistic deposition as a tool for development, an evaluation of the performance characteristics of HFA 134a and low-GWP propellant 152a was performed using APSD and INVIDA services.
Canisters containing API formulated with HFA 134a and HFA 152a were provided by Koura Global (Cheshire, UK) and had target doses of 100 mcg of albuterol sulfate. Testing used three devices and six replicates per device of each formulation. The tests performed included:
- Single actuation content (SAC)
- APSD using a next-generation impactor (NGI)
- Human-realistic regional deposition and delivered dose with INVIDA services.
Manual shaking and actuation conditions were performed for all samples.
SAC – Total Net Content Recovery
SAC samples were collected for both formulations using the same standard albuterol sulfate collection, extraction and high-performance liquid chromatography (HPLC) protocol. The target API dose for each formulation was 100 mcg.
Figure 2 shows similar results for both propellant formulations, with most data points falling within the acceptance range of ±15%. However, low-GWP propellant 152a showed a higher degree of variation and some outliers. Average recovery values were well within the acceptance range, with 95.3 mcg for 134a and 98.2 mcg for 152a. Similar results from this test consistently showed that both formulations delivered the same amount of API. This established a baseline for delivered dose and allowed for comparison of APSD and INVIDA.
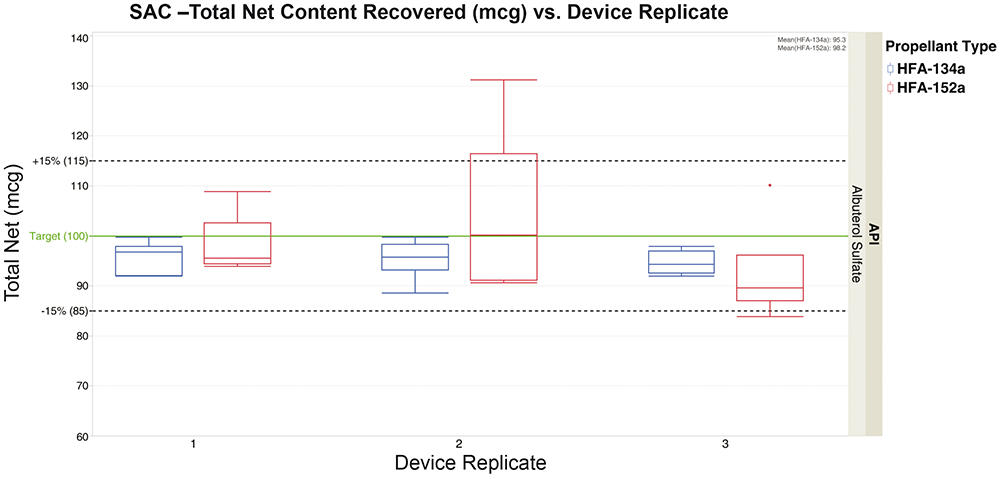
Figure 2: SAC – total net content recovery of albuterol sulfate (mcg) of formulations containing 134a and 152a propellants.
APSD – Total API Recovery from NGI
APSD was performed using an NGI and US Pharmacopoeia induction port at a constant flow rate of 30.0 L/min. Each data point consisted of one actuation and a standard eight-stage extraction, with standard HPLC used for quantification.
Figure 3 shows that both formulations have similar performance across all stages. The vast majority of API was deposited in the induction port.
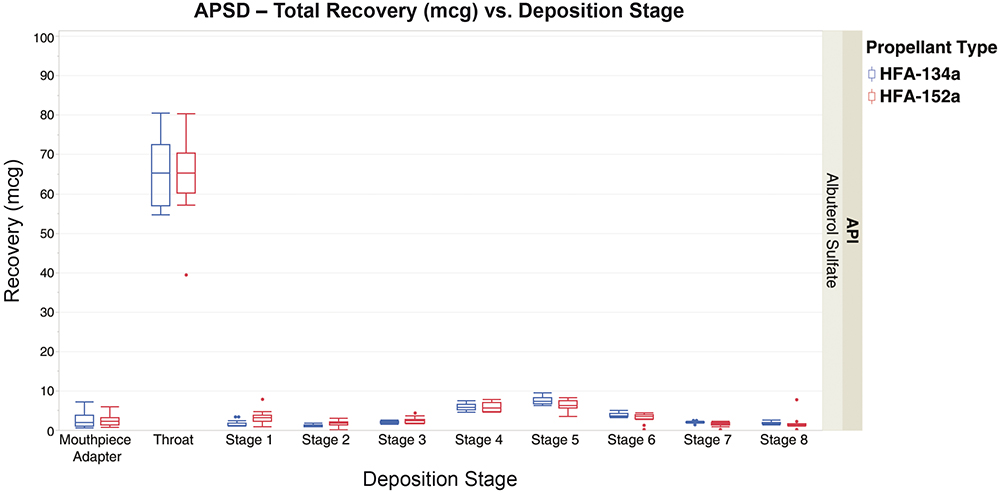
Figure 3: APSD – total albuterol sulfate recovery from NGI stages for formulations containing 134a and 152a propellants.
Figure 4 excludes the induction port, allowing for a more focused look at the mouthpieces and stages 1–8. Again, similar performance is seen across all stages for both propellant formulations. While variation is seen for both propellant formulations, 152a showed more variation overall.
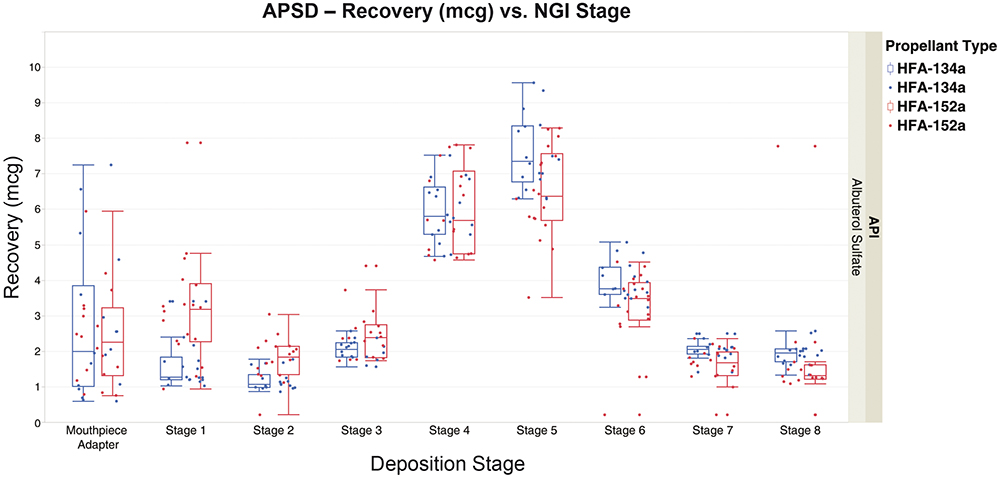
Figure 4: APSD – total albuterol sulfate recovery from NGI stages for formulations containing 134a and 152a propellants, excluding induction port.
INVIDA – Regional API Recovery
Regional deposition with INVIDA services used a breathing profile that represented a normal healthy adult male, obtained from published data.3 API recovery was performed for the mouthpiece adaptor and the three regions: mouth-throat, trachea and lungs.
Figure 5 shows similar performance for both propellant formulations in all the regions. Most of the API was deposited in the mouth-throat and lungs, with little deposition seen in the mouthpiece adaptor and trachea. Variation was observed in both propellant formulations, with 152a showing more outliers.
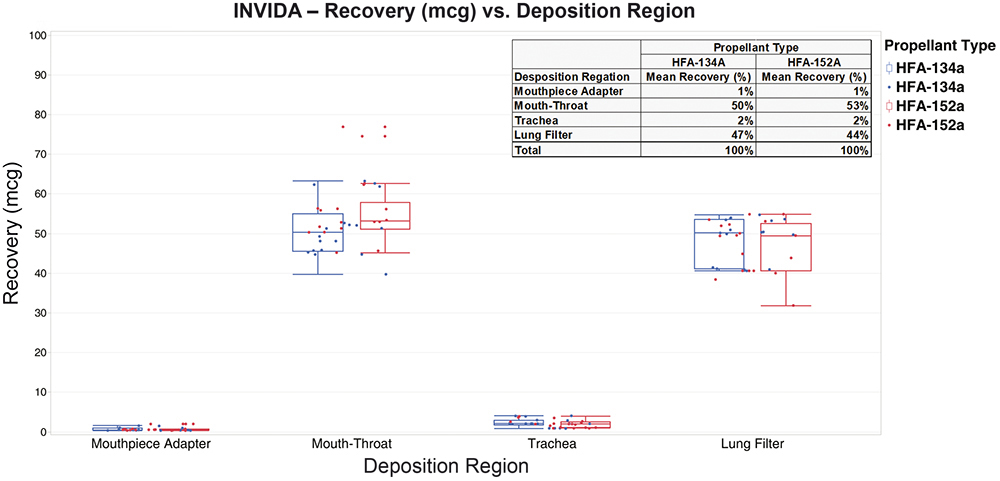
Figure 5: INVIDA regional recovery and relative delivered dose for formulations containing 134a and 152a propellants.
“When compared with traditional APSD, INVIDA services
highlighted the same performance differences in a fraction of the time.”
SUMMARY OF FINDINGS
- Results from SAC, APSD and INVIDA show similar performance for 134a and 152a propellants
– HFA152a resulted in slightly more variable output compared with HFA134a across all tests - Minor differences in regional deposition when comparing APSD with INVIDA can be expected, given the following differences:
– Constant flow rate for APSD and human-realistic breathing profile of INVIDA – 90° angle versus human-idealised mouth-throat model
– The regional breakdowns are not one-to-one between the APSD and INVIDA - INVIDA technology showed comparable performance between the propellants in a fraction of the time compared with APSD.
CONCLUSION
APSD is an essential test for pMDI development and approval that provides an indication of overall product performance. However, its considerable time requirements limit the speed of development and increase costs. To accelerate development, pMDI development and reformulation, the industry needs a tool for rapid evaluation of product performance that provides the same insights as traditional methods. In this study, Proveris Laboratories has illustrated the use of human-realistic regional deposition to support traditional development processes.
Similar results from SAC, shown in Figure 2, established a delivered dose baseline that allowed for comparison of APSD and INVIDA results. Both APSD and INVIDA identified the same performance for unoptimised formulations containing 134a propellant and a 152a low-GWP propellant. These techniques showed that both formulations performed similarly and that the 152a formulation had more variable results. One of the differences between these two techniques is the time required – when compared with traditional APSD, INVIDA services highlighted the same performance differences in a fraction of the time. Comparing time requirements for sample collection in Figure 6 shows a fivefold increase in throughput for INVIDA when compared with APSD. This represents a considerable time saving that would be even greater in a large-scale study.
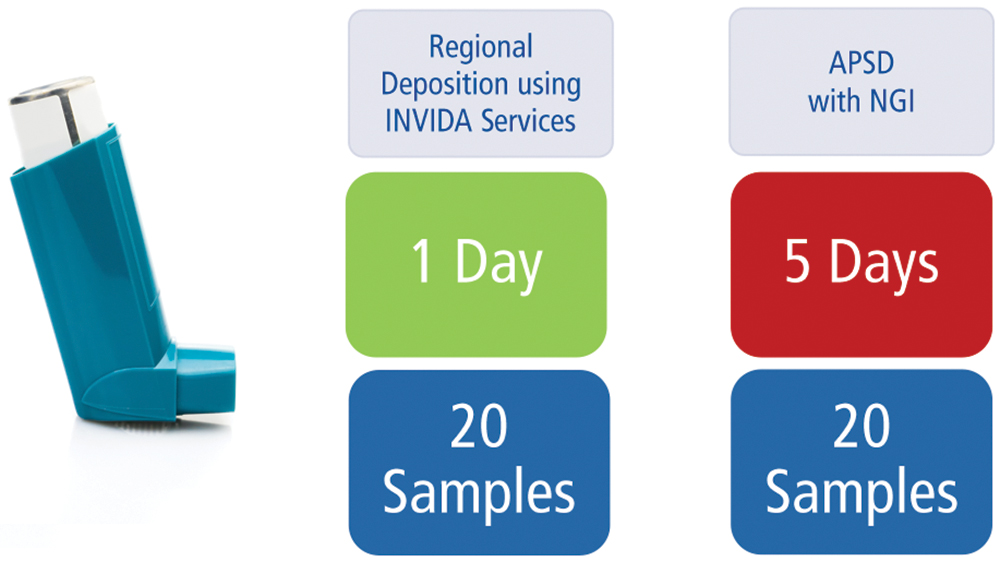
Figure 6: Time required for pMDI sample collection using INVIDA and APSD for 20 actuations.
Given similar findings and time savings provided by INVIDA services, a workflow that minimises dependence on APSD would be advantageous. Using INVIDA services for extensive early-development testing, such as product viability, device formulation screening and initial indications of equivalent performance, can provide the speed to knowledge needed to make informed decisions quickly. After development using INVIDA, pharmaceutical developers can be confident that APSD data for submission will resemble INVIDA data. Rapid performance indications provided by INVIDA services allow developers only to use APSD when necessary, reducing development time and cost.
This work would not have been possible without the expertise of Naveen Madamsetti, Senior Application Chemist, and Ellen Krett, Application Chemist, both at Proveris Laboratories, in generating the study data, and Koura Global, which provided inhaler propellant formulations for testing.
REFERENCES
- “U.S. Ratification of the Kigali Amendment”. US Department of State, Sep 21, 2022.
- United Nations Treaty Collection, Web Page, accessed Oct 2023.
- Delvadia RR et Al, “In Vitro Tests for Aerosol Deposition. IV: Simulating Variations in Human Breath Profiles for Realistic DPI Testing”. J Aerosol Med Pulm Drug Deliv, 2016, Vol 29(2), pp 196–206.

