To Issue 151
Citation: Schnitzler I, “Extending Patient-Friendly Drug Delivery to High-Volume Wearable Devices”. ONdrugDelivery, Issue 151 (Sep 2023), pp 52–56.
Iris Schnitzler discusses the benefits of on-body delivery systems, including the flexibility and convenience they bring to patients managing chronic conditions, and how the Sorrel platform overcomes some of the inherent complexities and challenges of developing such a device. Dr Schnitzler then goes on to speak with Bas van Buijtenen and Andrei Yosef about the Sorrel platform and how its integration within LTS supports the company’s fundamental objectives of enabling partners and empowering patients.
While the idea of being able to address the multiple challenges of on-body drug delivery with a one-size-fits-all solution would certainly have mass appeal among pharmaceutical companies, this simplified vision does not reflect the true complexity involved in the development of wearable device technology.
“The breadth of variables at play requires pharmaceutical companies and their partners to hit a vast number of specific targets unfailingly to bring a product or device to market successfully.”
In reality, the breadth of variables at play requires pharmaceutical companies and their partners to hit a vast number of specific targets unfailingly to bring a product or device to market successfully. And in a world where most new molecular entities are biologic in nature, and where increases in viscosities and volume to be infused are challenging conventional delivery devices, decisions about formulation, delivery platform and administration method must be addressed both individually and then as part of a complete solution.
Further to the content of a drug product, the method of delivery must also accommodate context. It is vital to support the needs and wishes of patients and maintain flexibility and comfort to increase the likelihood of compliance and adherence, and increasingly this means providing highly convenient, easy-to-administer delivery solutions that can be controlled directly by the patient themselves, either at home or via a transportable system that can fit with the fast pace of modern, mobile living. It is also important not to forget all the economic factors regarding device manufacture, assembly, storage and supply, all of which will collectively have a strong bearing on total cost.
These considerations all underline the tricky balancing act of establishing the optimal form of drug delivery for a given molecule, ensuring not only that it supports efficacy and meets regulatory standards, but also that it answers the critically important demands of the target patient population.
LTS’s approach to drug delivery has always been underpinned by a sensitivity to patient requirements. The philosophy that drives the company’s growth and fuels its innovation – We Care. We Create. We Deliver – is built on a belief of continued improvement in the experience of patients accessing essential medicines. This is evident in established platforms, such as transdermal patches and oral thin films, as well as emerging technologies, including micro array patches (MAP) for the transdermal delivery of large-molecule, biological actives. These methods all bring common benefits to patients in the form of simple, discreet and pain-free self-administration.
“The Sorrel platform will also benefit from being part of a strong market-leading brand that has global presence.”
Following the integration of Sorrel into the LTS group under the LTS Device Technologies division, this extensive portfolio now also includes a wearable injector platform that supports large-volume drug delivery through a vial or cartridge-based on-body device system (OBDS). This is an important expansion of LTS’s patient-friendly drug delivery capabilities, creating opportunities for wearables in a range of delivery applications.
The OBDS market has attracted significant attention in recent years because this type of device can bring flexibility and convenience to patients who are managing chronic conditions through regular injections. By simplifying and automating the injection process, OBDSs often represent an easier pathway for patients, which can improve adherence and lead to better outcomes.
For the pharmaceutical companies developing a combination product, flexibility and comfort at the patient level can, however, add complexity to the fill-finish process. For example, many current OBDSs require the pharmaceutical company to decide between a patient-friendly, prefilled, preloaded device that uses a proprietary cartridge or a pharma-friendly device that will accept standard ISO vials and commercially available containers, but which requires additional steps to be carried out by the patient during dose administration.
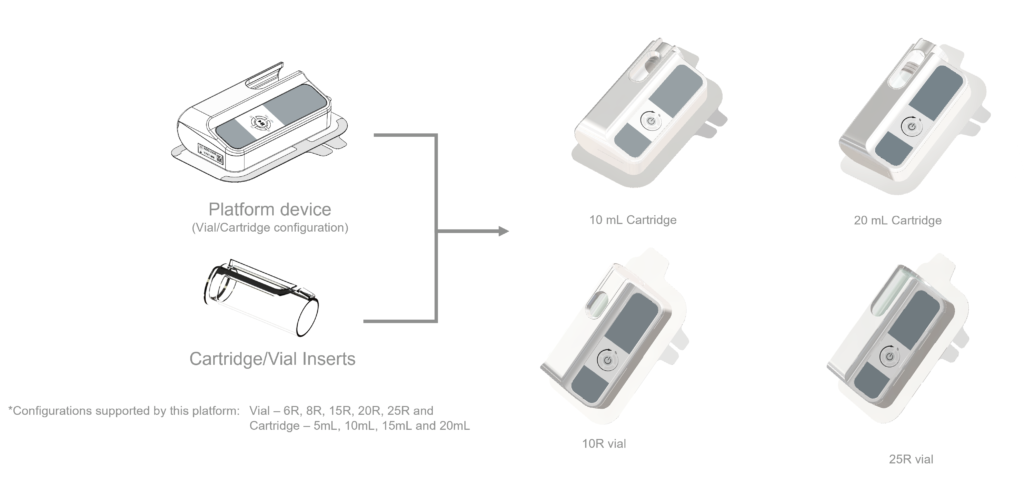
Figure 1: The Sorrel OBDS: an innovative platform design enabling customer flexibility during development.
The Sorrel platform addresses all these challenges in a market-ready vial or cartridge-based OBDS for the subcutaneous delivery of large-molecule drugs (Figure 1). The ergonomic, user-friendly system incorporates a disinfection chamber with an integrated UV-C LED, meaning it can be presented to a patient prefilled and preloaded, simplifying use and optimising uptake without having to replace the current primary container.
Here follows the Q&A with Bas van Buijtenen and Andrei Yosef (Figure 2):

Figure 2: Leadership in action: Bas van Buijtenen (left) and Andrei Yosef embracing a shared tomorrow.
Q What do you see as the key benefits of bringing the Sorrel platform to LTS?
AY Looking at it from the perspective of the device technologies division (formerly Sorrel), the ownership of LTS brings real strength by facilitating investment and supporting growth at a much faster rate. The platform has passed an inflection point in its growth. The first combination product containing our device has imminent US FDA approval, with several drugs close behind in the clinic. So, by facilitating investment and supporting our growth, being part of LTS will help us expand our manufacturing footprint and “future proof” ourselves for expected expansion as more of our in-clinic partnered programmes commercialise.
The Sorrel platform will also benefit from being part of a strong market-leading brand that has global presence. It will mean we can offer a more “turnkey solution” for our pharma partners in addition to our device platform.
“Pharma companies will seek to qualify solutions from their suppliers on many fronts before committing to bring them to market commercially.”
BvB At LTS, we have a long-standing passion to bring convenient drug delivery solutions to our pharmaceutical customers and their patients, evidenced by the billions of transdermal patches and oral thin films that we have safely supplied over the years. Bringing that ease of administration to the world of large molecules has been a central part of our strategy, as can be witnessed through our innovation in MAP technology. The addition of the Sorrel business accelerates that strategy and brings our customers access to an entirely new technology. It allows LTS to take a big step forward into the world of biologics and biosimilars, bringing greater balance to our product portfolio, and opening up tremendous potential for further innovation.
Q Can you elaborate on what this means to your pharmaceutical partners?
AY Over the last year we have installed and validated two semi-automated production lines, which brings our commercial-ready capacity to 1.5 million units per year (Figure 3). We have engineered a very efficient, scalable manufacturing process and, with the acquisition, we now have the potential to increase this output significantly and at multiple sites all over the world.

Figure 3: LTS on-site manufacturing facilities: two new semi-automatic lines for a more efficient and scalable manufacturing processes.
Beyond investment in innovation and capacity growth, our capabilities have also been broadened through the acquisition, allowing us to serve our partners more fully. We are extremely excited to offer pharmaceutical companies a solution for the assembly of primary containers into the device and final packaging. We can place this technology at our partners’ facilities or manage the entire process within LTS facilities in the EU or US.
BvB Pharma companies will seek to qualify solutions from their suppliers on many fronts before committing to bring them to market commercially. A lot of that is, of course, about the technology: does it meet our technical requirements and has that been validated and documented robustly? How well does it do on human factors? Next to that, our customers need to trust the organisation: does it have the talent, the capabilities, the quality mindset, the capacity, the capital and commitment to tie it to the fortunes of a blockbuster drug? With the progress that the Sorrel technology has made, and with the broader LTS organisation’s backing, we will help our customers tick all of those boxes.
Q What are your future ambitions in terms of technology?
AY As part of our strategy, and as a market leader, we need to keep driving innovation. We have just completed clinical evaluation of our new platform device featuring a unique insert module. This new platform device offers a range for both vials (6–25 R) and cartridge (5–20 mL) while using exactly the same device. This will allow our pharmaceutical partners with a large pipeline of drugs to focus on one device only that can serve most of their drug needs. Not only does this allow for faster time to market but also even faster time to clinic.
BvB We will continue to innovate around these platforms, guided by the LTS mission of “We Care. We Create. We Deliver”. What the platforms have in common at a fundamental level is that they are geared to making patients’ lives easier, creating access to immunisation and therapies more quickly and minimising disruptions to patients’ lives. Both our MAP and OBDS technologies are at a stage where they have demonstrated consistent clinical performance, making them ready for commercialisation and scale-up. We are closest to market entry with our wearable devices, where our first customer is anticipating a commercial launch before the end of the year. Our teams are working on a rich pipeline of opportunities for wearables and MAPs to help more customers follow in those footsteps. In each of those projects, customers benefit from the foundations that have now been laid, de-risking and accelerating all future projects.
Q Will growth at LTS continue to be supported by acquisitions in the future?
BvB To clarify, LTS is not a company that makes acquisitions for the sake of it. Our growth strategy is clear and for every element of that strategy there is an organic action plan. Mergers and acquisitions are effectively a lever we can pull to accelerate that underlying strategy.
In recent years, we have used it to fulfil two strategic priorities. The first was strengthening our position in North America, with the acquisition of our Saint Paul (MN, US) site facilitating a major step forward for LTS by giving us, among other capabilities, an R&D facility in that market.
Now, with the acquisition of the Sorrel business, we have accelerated another growth priority: bringing breakthrough technologies to the world of large molecules, and thus extending our portfolio of patient-friendly solutions. Our next priority is, of course, to integrate these businesses in such a way that we maximise their organic contribution and make them part of the foundations upon which new growth can be built. Innovation breeds further innovation.
In conclusion, while wearable devices and OBDSs must be as simple as possible from a user’s perspective, these are highly sophisticated platforms that are evolving to meet complex drug-delivery challenges, for everything from small-molecule therapies to large-volume biologics and biosimilars. It is less a case of one size fits all and more a case of employing the right platform to reach the market in the most effective way. With the addition of Sorrel’s vial or cartridge-based OBDS, LTS has the breadth of platforms to deliver on this promise for pharma partners and patients alike.
Find out more about LTS’s Sorrel device technology at: www.ltslohmann.com/en/our-technologies/sorrel.

