Citation: Simpson I, “How Connectivity Adds Value to Injectable Drug Delivery Devices”. ONdrugDelivery Magazine, Issue 101 (Oct 2019), pp 60-64.
Iain Simpson discusses why – in the context of the growing shift in emphasis from improving adherence to the better outcomes that can arise from doing so – connectivity is becoming increasingly important in injectable drug delivery, and how it can benefit patients, providers and pharmaceutical manufacturers.
“Clinical trial data might be sufficient to get a drug approved as safe and effective but may not satisfy the needs of payers. They may demand further trials post approval to assess the value of medication in real-world conditions.”
Medication non-adherence is a major issue and represents a barrier to effective healthcare, with the WHO reporting in 2015 that medication adherence among patients with chronic diseases averages only 50% in developed countries.1 The problem has long been recognised. In 1985, former US Surgeon General C Everett Koop, MD, succinctly observed, “Drugs don’t work in patients who don’t take them.”2 Yet over the following 30 years or more, frustratingly little progress has been made in reliably tracking and successfully increasing medication adherence.
However, the arrival of widespread and increasingly low-cost connectivity (the so-called Internet of Things), coupled with the pervasive use of smart devices such as phones and tablets, now creates an opportunity to change this. Furthermore, the use of connectivity has the potential to demonstrate a direct link between medication adherence and healthcare outcomes. The emergence of the IoT is ushering in a new era of precision medicine and value-based payments – approaches that pave the way to a more personalised and effective healthcare system.
MULTIPLE FACTORS INFLUENCE ADHERENCE
Part of the challenge is that multiple economic and behavioural factors affect adherence. These vary depending on the drug, disease and delivery method, but include patient concerns about side effects, questions about the efficacy of the drug, difficulty using the delivery device properly, insufficient patient education and engagement, and high out-of-pocket costs.
Non-adherence has significant ramifications, both clinically and economically. For patients, it can result in additional hospitalisations and medical visits, contribute to poor health outcomes or even death, reduce productivity and compromise quality of life. It also significantly burdens healthcare systems,3 with estimates of avoidable costs ranging up to US$290 billion (£233 billion) in the US and €1.25 billion (£1.12 billion) in Europe.4 However, accurately estimating the true magnitude of the economic impact on healthcare costs is difficult, due to varying study methodologies and the absence of standardised measures.4
“A step beyond value-based healthcare is recognising that patients are not all the same, so prescribing the same dose to all may not be optimal. We need to move toward precision or personalised medicine.”
Pharmaceutical companies are also affected by non-adherence, losing an estimated 36% in potential sales per drug on average.5 This adds up to approximately $188 billion in annual losses for the US pharmaceutical industry alone.6
In the past decade, healthcare has become increasingly digitalised with the implementation of electronic health records (EHR) in many markets, connected devices and equipment in hospitals and laboratories, and smartphones and tablets to support healthcare professionals. At the same time, prescription medication – especially for patients with chronic diseases – is increasingly being taken at home rather than in the hospital, underscoring the need to explore and expand mobile digitalisation outside the hospital as well as within.
Fortunately, the widespread availability of low-cost connectivity such as Bluetooth® and near universal access to mobile technologies such as smartphones have opened the door to new opportunities in this arena. With smartphones already integral to most patients’ lives across the globe, the logical next step is to use them as tools to support both medication adherence and disease management.
Belief in the potential of connectivity to improve medication adherence through better usability, medication reminders, remote patient support and other functionalities that support behavioural change has been a primary driver in the development of connected drug delivery devices.
SHIFTING THE FOCUS FROM ADHERENCE TO OUTCOMES
Pharma companies typically establish that drugs are effective by demonstrating therapeutic benefit in controlled clinical trials. However, the outcomes from these studies might be considered “best case” and don’t necessarily guarantee similar results under real-world conditions, where complications such as disease comorbidities, variations in treatment pathways, poor education and healthcare funding adversely impact performance. Clinical trial data might be sufficient to get a drug approved as safe and effective but may not satisfy the needs of payers. They may demand further trials post-approval to assess the value of medication in real-world conditions. One approach to resolving this dichotomy is to make clinical trials more representative of real-world treatment, but that risks losing some of the potential offered by new treatments. A better approach is to understand how to address some of the real-world health challenges, such as non-adherence, more effectively.
“Connected drug devices can generate reliable data in near real time, allowing providers, payers and pharma companies to understand what works and what doesn’t. Medication usage data collected directly from devices
and integrated with electronic patient reported
outcomes (ePROs) and digital biomarkers can be used
to understand medication performance under
real-world conditions as well as monitor treatment performance on individual and cohort levels.”
Where reimbursement is driven by payment by use, improving medication adherence provides a direct benefit to pharmaceutical companies through increased drug sales and underpins much of their investment in connected delivery systems. It also suggests a better patient experience is making it easier to overcome some of the barriers that negatively impact adherence. However, in isolation, improved adherence is less well aligned to the interests of payers and providers, who are concerned about healthcare outcomes, costs and efficiency.
While it is reasonable to assume that increasing adherence ought to improve health outcomes and move the dial back towards the “best case” results from clinical trials, demonstrating a direct link has proven difficult. Clinical trials have been reported that show a positive impact on outcomes from improved adherence, yet significant doubts remain as to whether this success will scale to real-world healthcare. The cost and complexity of scaling the technology, combined with the real-world healthcare challenges described above, can mask or reduce the benefit. Until these challenges are addressed and more compelling real-world evidence is generated, the likelihood and extent of reimbursement will be limited.
But, as the healthcare industry continues its march towards a value-based payment model in lieu of a volume-based one, the landscape is set to change. Aligning patient, provider and pharma company needs with the healthcare reimbursement process is critical and probably best accomplished by shifting the focus from adherence alone to improving outcomes, as shown in Figure 1.
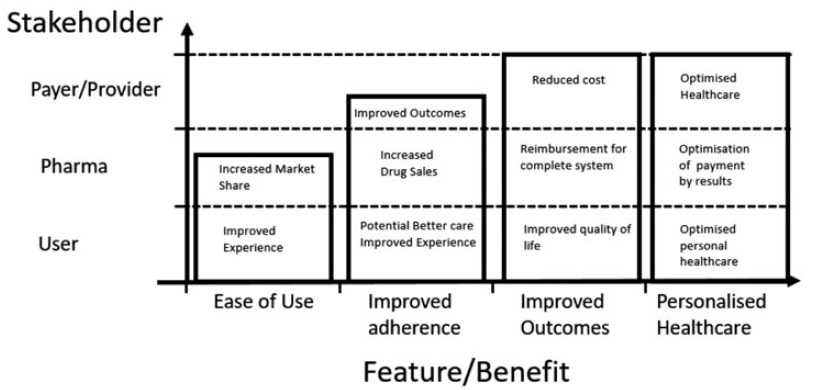
Figure 1: Benefits of electronic and connected devices.3
Adherence is only one part of the story. Developing the quote above from C Everett Koop, it can also be said that drugs don’t work in patients who don’t respond to them. So, a poor outcome for a therapeutic intervention could be a result of non-adherence and/or non-response. A step beyond value-based healthcare is recognising that patients are not all the same, so prescribing the same dose to all may not be optimal. We need to move toward precision or personalised medicine, where treatment is tailored to individual needs. At present, relatively few drugs beyond insulin used to treat diabetes, are titrated.
As well as providing a better patient experience, connected drug devices can generate reliable data in near real time, allowing providers, payers and pharma companies to understand what works and what doesn’t. Medication usage data collected directly from devices and integrated with electronic patient reported outcomes (ePROs) and digital biomarkers can be used to understand medication performance under real-world conditions as well as monitor treatment performance on individual and cohort levels.
In addition, these data can be integrated into EHRs, which help aggregate diagnostic data, prescriptions, treatment adherence and outcome measurements. These digital advantages provide a two-fold benefit: the ability to support outcomes-based payment accurately and to deliver more responsive, personalised healthcare.
Looking toward the future, moving from clinical assessments and questionnaire-based ePROs to smartphone-connected tracking that monitors patient activity, sleep and other behaviour will further quantify medication efficacy. In addition to supporting reimbursement, this data can help healthcare providers tailor and improve the effectiveness of their patient treatment plans – assuming the data can be provided to professionals in an actionable form.
REAL-WORLD RESULTS FROM CONNECTED INJECTORS
Despite the potential benefits, development and adoption of connected drug delivery devices has been relatively limited so far. Typically, connected injection devices are being used for high-value medications for chronic diseases in competitive markets such as multiple sclerosis (MS), human growth hormone deficiency and diabetes, which often already have patient support programmes in place. Integrating connected devices into these existing programmes supports improved patient engagement, enables more evidence-based patient guidance and allows providers to zero-in on patients most in need. However, scaling these interventions and applying them to wider healthcare practice remains challenging.
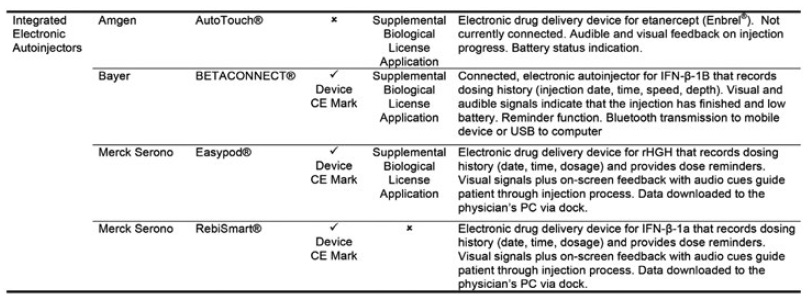
Figure 2: The four approved drug-device combination products using electronic autoinjectors.3
Four drug-device combination products using electronic autoinjectors have currently been approved for patient use (Figure 2). A quick look at the BETACONNECT™ Electronic Autoinjector (Bayer, Leverkusen, Germany), developed by Phillips-Medisize, illustrates the advantages of connected autoinjectors. The first connected electronic autoinjector approved in both the US and Europe for the delivery of interferon beta-1b for MS, the BETACONNECT system comprises an electronic autoinjector, the myBETAapp™ for patients and the BETACONNECT Navigator, a clinician dashboard (Figure 3).
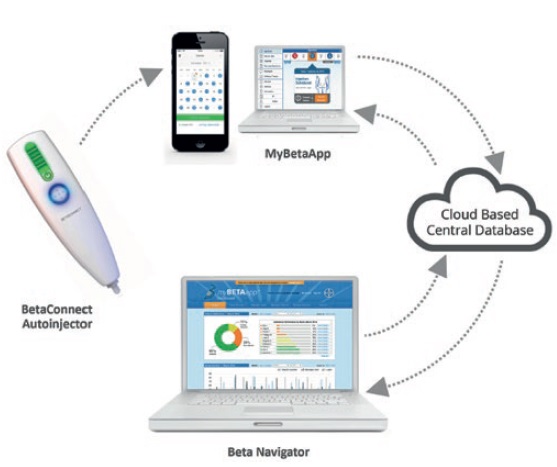
Figure 3: The BETACONNECT system for the treatment of MS.
The connected, patient-centred design offers:
- Ergonomic design with a button in the middle of the device that can be conveniently operated with one hand
- Adjustable injection speed and depth, plus automatic needle insertion and retraction
- Bluetooth connectivity that ensures data on injection time, volume and body location sync with the patient app and clinician dashboard, either wirelessly to a mobile device or via USB to a computer
- Personalised, localised messages and reminders for patients on their device and in the app
- A wellness tracker where patients can record their well-being and selected health parameters.
Research results have been positive. Nearly three-quarters of participants were still using the autoinjector and nearly 60% achieve adherence levels above 80% by the end of one study.7 In another study of the myBETAapp, 93% of those who used the app considered it helpful and 87% said it supported their therapy.8
As this emerging evidence demonstrates, BETACONNECT has been well-received by patients and can improve medication adherence. However, outcomes-related evidence remains limited, with no head-to-head study data yet published that might show the incremental benefit of connectivity and digital service. Further studies are being conducted to evaluate long-term adherence as well as treatment outcomes in order to support reimbursement and better identify patients at increased risk of exacerbations or relapses so that timely intervention can be provided.
The pioneering model of BETACONNECT and other current connected health solutions, which link a drug delivery device to an app and cloud data system for a particular purpose such as providing drug usage monitoring or supporting disease management, has opened the door to much wider use of the technology. The approach has been shown to work well for current business models operating in conjunction with patient support programs, demonstrating the value of the collection of quantitative and actionable data. Once technical, clinical and commercial challenges are overcome, it is also creating an infrastructure that can be utilised more widely. This will allow integration of the approach into healthcare, scaling of the solution to larger real-world patient populations and create the ability to address more complex disease management situations, involving multiple medications and comorbidities.
Models that solely rely on pharmaceutical companies getting a return on their investment through increased drug sales, and not through reduced healthcare costs, need to evolve to realise the wider potential of the approach in addressing non-adherence and improving other aspects of healthcare. Greater integration with outcome measures, analytics and the wider healthcare system will enable future connected devices to more effectively engage all the key healthcare stakeholders, including patients, payers, clinicians, pharma companies and technology providers.
CAPITALISING ON CONNECTED HEALTH EXPERIENCE & EXPERTISE
“Models that solely rely on pharmaceutical companies getting a return on their investment through increased drug sales, and not through reduced healthcare costs, limits the wider potential benefits of the approach.”
Trailblazers who are willing to invest in the development of additional innovative connected injectable drug devices have a unique opportunity to create a win-win situation for all stakeholders. As with any development project, the right partner is key.
Phillips-Medisize is one of the few companies that has partnered with pharma manufacturers to bring a connected injectable drug delivery system to market successfully. Its end-to-end development and manufacturing capabilities streamline design and production, speeding time to market. In addition, the company’s deep knowledge and experience regarding different rules and regulations across global markets enables proper configuration of the product to help reduce project costs and support regulatory approval.
In addition, Phillips-Medisize’s ability to manufacture the electronics internally makes connectivity for drug delivery devices far more affordable, supporting wider deployment.
In the four years since BETACONNECT came to market, the company has also created a highly scalable connected health platform that offers numerous benefits, including comprehensive information-sharing and analytics, robust cybersecurity and a modular approach that can be easily customised to multiple therapeutic areas. The company plans to introduce an app framework that can be tailored to link patients and devices to the platform in a wide range of disease areas. This configurability can reduce the regulatory burden of needing to have the solution re-approved for each new application.
CONCLUSION
Increasingly, evidence demonstrates that connecting injectable drug delivery devices results in higher levels of patient medication adherence and persistence longer term.
Consistently high adherence certainly adds value, since medications cannot help patients who don’t take them. The next challenge, however, is to generate databased evidence that better adherence improves outcomes. Although achieving this goal presents challenges, connected injectable drug delivery devices are already playing a critical role in capturing reliable real-world data on medication usage, supporting better decision-making, and more effective use of relatively expensive resources in areas such as patient support programs and healthcare pilot studies. This work is building the infrastructure as well as the evidence base for the linkage between adherence and outcomes, enabling better alignment of reimbursement to the real-world value a therapeutic brings. In turn, this can enable new business models that can support wider uptake of digital technology in healthcare. But most importantly, combined with patient-reported outcomes and digital biomarker data from wearable biosensors, connected drug delivery devices have powerful potential to improve outcomes and quality of life for patients living with chronic disease.
REFERENCES
- Lam WY, Fresco P, “Medication Adherence Measures: An Overview”. BioMed Research International, 2015, Vol 2015, Article ID 217047.
- Lindenfeld J, Jessup M, “Drugs don’t work in patients who don’t take them”. Eur J Heart Fail, 2017, Vol 19(11), pp 1412-1413..
- Dean K, Simpson I, “Designing Connected Devices for Inhalation: Lessons Learned from Injectables”. Proc RDD Eur 2019, pp 109-122.
- Cutler RL et al, “Economic impact of medication non-adherence by disease groups: a systematic review”. BMJ Open, 2018, Vol 8(1), e016982.
- “IMS Special pharma strategy supplement: Reviving the primary care market”. Research Report, IMS Health, July 2008 (accessed through Capgemini “Patient Adherence: The Next Frontier in Patient Care”).
- “Estimated Annual Pharmaceutical Revenue Loss Due to Medication Non-Adherence”. Research Report, Capgemini Consulting HealthPrize Technologies, 2016.
- Kleiter I, “Adherence, satisfaction and functional health status among patients with multiple sclerosis using the BETACONNECT® autoinjector: A prospective observational cohort study”. BMC Neurology 2017, Vol 17(1), p 174.
- Limmroth V et al, “The BETACONNECT™ system: MS therapy goes digital”. Neurodegenerative Disorder Management, 2018, Vol 8(6), pp 399-410.

