Citation: Suman J, “How Evolving Patient Needs Have Fuelled the Development of Nasal Drug Delivery”. ONdrugDelivery, Issue 119 (Apr-May 2021), pp 18–22.
Julie Suman considers how the needs of the patient have driven advances in nasal drug delivery and looks at the latest developments and benefits of this method of drug delivery.
“Innovation by drug device development partners such as Aptar Pharma is therefore crucial to the continued success of pharmaceutical companies in delivering better outcomes via more patient-friendly techniques.”
The development programme for every new drug or medical device is driven by one overarching objective: creating safe and effective medications that improve patient outcomes.
However, while this might be the clear aim that guides pharmaceutical partners on their path of continuous improvement and development, achieving it in practice is far from easy. The notion of patient outcomes is recognised as a complex construct that can be measured in various ways, and success is dependent on several interlinked elements being correctly aligned.1
Pharmaceutical companies are challenged to deliver this alignment by delicately balancing their primary goal − achieving a desired therapeutic effect − with several other factors, including patient adherence and compliance, which are heavily influenced by the method of drug delivery. Intravenous routes of administration, for example, may provide advantages in terms of rapid and accurate dosing, but less-invasive alternatives, such as oral or nasal drug delivery, are more readily accepted by patients and are also easier to administer by healthcare professionals (HCPs) and patients alike. Innovation by drug device development partners such as Aptar Pharma is therefore crucial to the continued success of pharmaceutical companies in delivering better outcomes via more patient-friendly techniques.
A BRIEF REVIEW OF THE BENEFITS OF NASAL DRUG DELIVERY
From the patient’s perspective, research has demonstrated a preference for less-invasive drug delivery methods such as intranasal administration. In a study examining patients’ responses between epinephrine delivered via a nasal spray or via an autoinjector, it was found that patients – all of whom suffered from severe allergies – reported a significant preference for nasal epinephrine, specifically citing factors including ease of learning and use, device portability, safety and comfort within their responses.2
As an accessible, non-invasive route to rival the pharmacokinetic profile of some intravenously administered drugs, nasal drug delivery is increasing in profile and potential. Advances in intranasal drug delivery technologies mean the benefits of this method are being brought to bear in an expanding range of therapeutic treatments, both local acting and systemic.
This is all made possible as a result of the biology of the nasal cavity, a highly vascularised area of approximately 160 cm2 in size.3 This large surface area provides the basis for relatively rapid drug absorption rates, leading to a fast onset of drug action. Unlike oral administration routes, nasal drug delivery also offers the advantage of bypassing the gastrointestinal tract, thus avoiding the risk of bioavailability being reduced as a result of first-pass metabolism.
Exploiting the benefits of nasal drug delivery is not new. In fact, the nose has long been recognised for its characteristics as a drug-administration pathway, as exemplified by the nasal insufflation of powders for local and systemic disorders in medieval Persia (Nofookh).4 Modern, Western medicine accelerated its interest in the mid-20th century, when early nasal drug delivery products were dominated by local-acting treatments for conditions such as nasal congestion and allergic rhinitis. Product examples include fluticasone propionate (Flonase, GlaxoSmithKline) and oxymetazoline hydrochloride (Afrin, Bayer Healthcare).
DEVELOPMENTS IN NASAL DEVICE TECHNOLOGY
As these products evolved into over-the-counter (OTC) remedies from their prescription origins, the convenience and ease of use of aerosolised delivery made them highly marketable to patients, particularly when contrasted with alternative methods for administering local-acting compounds. Nasal drops, for example, typically demand that the patient adopts a rather unorthodox head position, such as Mygind or Kaiteki, to achieve the gravitational conditions to support improved coverage in the nasal cavity. However, the discomfort and complexity associated with such manoeuvres can understandably lead to poor compliance among patients.
Such weaknesses have paved the way for metered-dose spray pumps to emerge as the dominant option for nasal drug delivery. Equally appropriate for drugs targeting local action as well as systemic absorption, and compatible with formulations in suspension as well as solution, these devices provide a platform for accurate, repeatable dosing and targeted delivery. A specified volume of liquid, between 50 and 140 μl, is contained within the device’s metering chamber and then aerosolised via compression of the actuator – following initial priming of the device. Releasing the actuator causes a pressure imbalance that triggers the refilling of the metering chamber via the dip tube.
During the spraying manoeuvre, make-up air enters the bottle, which potentially exposes the formulation to bacterial contaminants. As aqueous formulations may support bacterial growth, antimicrobial preservatives may be included within the formulation to maintain stability and prevent contamination throughout the product’s shelf life. The use of preservatives such as benzalkonium chloride (BAC), however, has led to concerns over their potential role in causing undesirable side effects such as damage to the ciliated tissue in the nasal cavity.5 While discussions continue on this contested subject, there has undoubtedly been a growing momentum behind preservative-free formulations, encouraged by the EMA’s advice that the threshold for BAC should be zero for all routes of administration, including nasal.6
In response, device manufacturers have developed innovative preservative-free intranasal spray systems. Aptar Pharma is no exception, with examples including its CPS technology platform and Advanced Preservative-Free (APF) system, which incorporate a filter membrane in the ventilation channel to prevent the ingress of any microbiological contamination with venting air. This ventilation channel is combined with a spring-loaded tip seal mechanism to eliminate the need for any preservatives or additives, such as silver ions or surface coatings. Rigorous integrity tests have proved that both the tip seal and ventilation channel protect against any ingress of bacteria from either air or liquid.
ENHANCING PATIENT CARE
For a growing number of applications, multi-dose nasal sprays are being replaced by devices that can deliver a controlled single dose (Unidose (UDS), Figure 1, left) or two doses (Bidose (BDS), Figure 1, right). Through their intuitive design, ease of use and no need for priming, such products allow for needle-free administration (or self-administration) of potentially life-saving emergency medicines where there is urgent need. Examples include naloxone (NARCAN®, Opiant) for treatment of opioid overdose and midazolam (Nayzilam®, UCB) and diazepam (Valtoco®, Neurelis) for treatment of seizures.
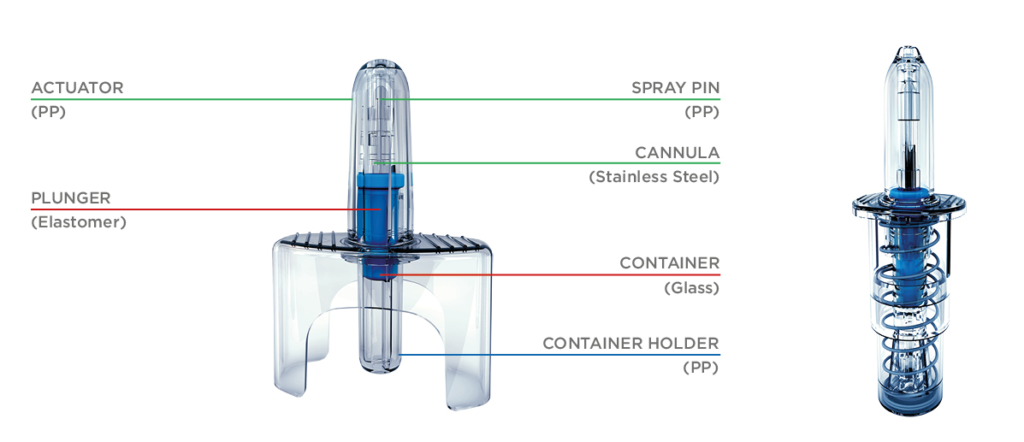
Figure 1: Unidose liquid (UDS) spray (left), and Bidose liquid (BDS) spray (right).
Further breakthroughs in recent years include esketamine nasal spray (Spravato®, Janssen Pharmaceuticals) for treatment-resistant depression. In addition, diabetic patients have benefited from the introduction of glucagon nasal powder (Baqsimi®, Eli Lilly and Company), which can be used in the event of a severe hypoglycaemic episode. Portability, ease of use and reliability of dosing are critical qualities in an application such as this, and a single-use nasally administered powder device represents significant advantages over the alternative option of a glucagon injection, which also requires refrigeration. This is a clear example of nasal drug delivery’s potential in the sphere of drug repurposing, where the reformulation of existing compounds can bring significant new advantages in terms of patient and caregiver accessibility. In addition to the treatment of diabetes, nasal powder also provides a delivery platform for migraine sufferers in the form of sumatriptan (Onzetra®, Avanir Pharmaceuticals). Such products exploit the unique advantages of nasal powders, which include their suitability for accommodating larger doses and supporting compounds lacking aqueous stability. In addition, a study of in vitro deposition has also shown that, when compared with an aqueous spray, nasal powder may improve targeting to the olfactory region (Figure 2).
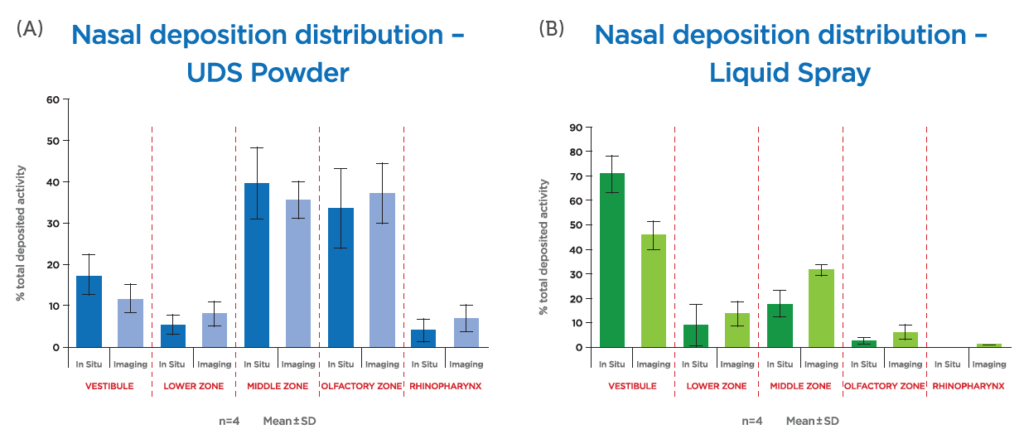
Figure 2: Nasal Powder Unidose device (UDSp) (A) gives higher deposition than Liquid Unidose device (B).
EMERGING OPPORTUNITIES FOR NASAL DEVICES
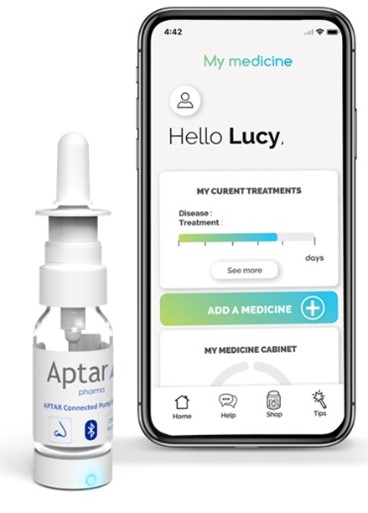
Figure 3. Connected devices are the future for digital healthcare.
As well as their intuitive design, nasal inhalation devices are subject to continued innovation by pharmaceutical companies and device partners to enhance the care and support provided to patients. With emergency therapies, for example, training kits have been developed that closely mirror the real-life experience of using a nasal spray with a view to optimising compliance and decreasing the mortality risk. Patient safety is also being addressed through the inclusion of lock-out mechanisms on devices, while the development of laterally actuated nasal sprays provides greater levels of comfort and simplicity for certain patient populations.
Dose counters have long been recognised as benefiting adherence, and rapid advances in technology have built on this platform to create an ecosystem for connected devices that play a more “active” role in supporting greater adherence. By monitoring, recording and communicating data on usage via the internet, connected devices represent a potent opportunity for establishing an ecosystem where pharmaceutical company, patient and healthcare practitioner are more closely integrated (Figure 3). Apps can prompt patients to take their medication, healthcare professionals can accurately track progress and pharmaceutical companies can receive anonymised data on real-world medicines use. In this scenario, avoiding medicines wastage is an obvious benefit, but it also represents a fundamental opportunity for delivering better long-term health outcomes.
Further high-value opportunities exist in the OTC space, where there is growing evidence of the benefits of nasal saline in promoting well-being and addressing the symptoms of air pollution, which causes the deaths of an estimated seven million people worldwide every year.7
OVERCOMING THE BLOOD-BRAIN BARRIER
At the other end of the spectrum, nasal drug delivery is demonstrating success at treating neurological conditions via the central nervous system (CNS) (Figure 4). It has been shown that targeting drugs at the roof of the nasal cavity enables molecules to be transported to the CNS via the olfactory or trigeminal pathway.8 Many existing therapies targeting these conditions suffer from poor bioavailability due to the blocking effect of the blood-brain barrier (BBB). In bypassing the BBB, intranasal delivery methods can be used with targeting strategies to increase the level of drug reaching the intended site of action.
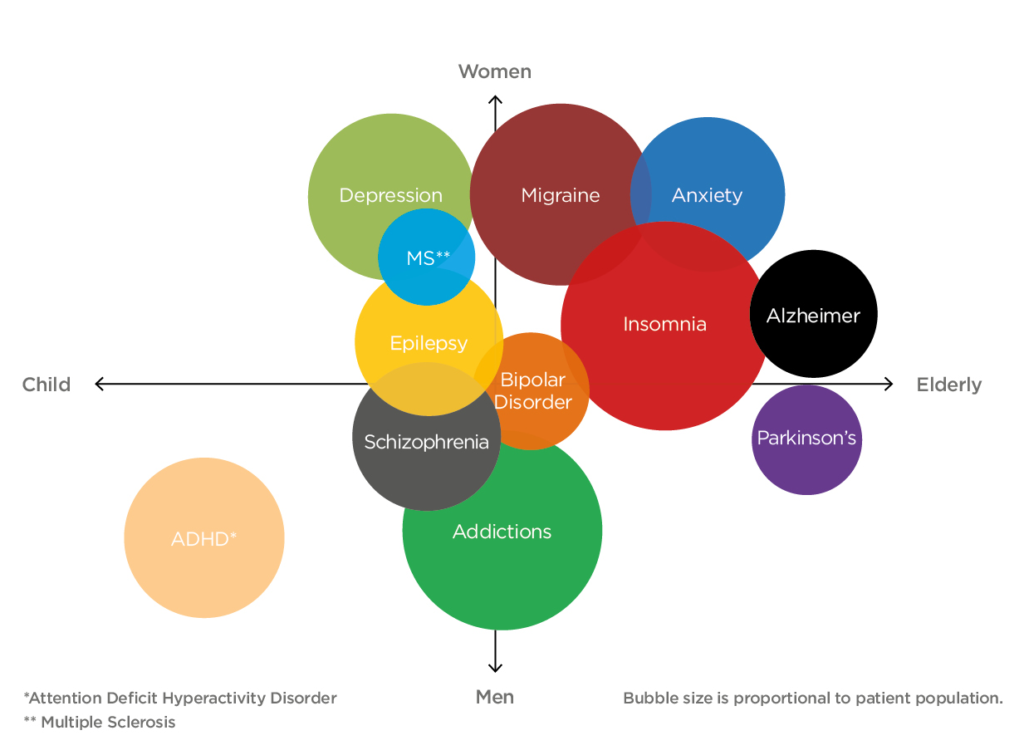
Figure 4. Unmet CNS disease states span across all demographics. The size of the bubble represents the size of the market for the disease state.
“At the other end of the spectrum, nasal drug delivery is demonstrating success at treating neurological conditions via the central nervous system (CNS). It has been shown that targeting drugs at the roof of the nasal cavity enables molecules to be transported to the CNS via the olfactory or trigeminal pathway.”
As research into this important area continues, nasal devices continue to be deployed in a prophylactic capacity through the delivery of vaccines. In the 2019–20 season, over three million children were vaccinated against flu across the UK with the live attenuated influenza vaccine (LAIV)9 nasal spray. Immunity is achieved through interaction with immune modulators in the nasal cavity and dendritic cells in the nasal epithelium. With vaccines in development for conditions including anthrax, HIV and Hepatitis B, among others, further opportunities exist in this area, where sufficient activity can be achieved by overcoming challenges in drug absorption, mucociliary clearance, the mucus barrier and enzymatic degradation at the nasal mucosa.
Today, the more immediate potential for nasal drug delivery concerns treatments for covid-19, which has rapidly emerged as the most urgent healthcare challenge of modern times. Promising treatments are currently in development that eradicate the virus from the upper respiratory tract while blocking the ACE-2 receptor, essential for the virus to infect cells. Examples of nasally administered treatments include antibodies such as immunoglobulin G (IgG) and immunoglobulin Y (IgY). According to the WHO, at least five nasal spray vaccines are now in early clinical trials. In tandem with mass vaccination programmes, nasal devices may enable therapies such as these to play an important part in our protection against covid-19 in its various forms in the years to come.
Nasal drug delivery has evolved into a highly versatile method of administration for locally acting and systemic drugs; for administering emergency doses and managing long-term conditions; and, in the case of preventative vaccines or breaking new ground, in challenging therapeutic areas. Central to all these diverse applications is the fact that patient outcomes have been enhanced by delivering medicines and therapies in a way that is more accessible and acceptable to the patient, smoothing their access to the care they need. With continued innovation, driven by pharmaceutical companies and their drug delivery partners, nasal administration is poised to play an ever-more important role in tackling unmet patient needs in the future.
All images courtesy of Aptar Pharma.
REFERENCES
- Liu Y et al, “Patient outcomes in the field of nursing: A concept analysis”. Int J Nurs Sci, 2014, Vol 1(1), pp 69–74.
- Beckman S, “Patients prefer epinephrine nasal spray over auto-injector”. Healio.com, November 5, 2019.
- Gizurarson S, “Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery”. Curr Drug Deliv, 2012, Vol 9(6), pp 566–582.
- Zarshenas MM et al, “Nasal Drug Delivery in Traditional Persian Medicine”. Jundishapur J Nat Pharm Prod, 2013, Vol 8(3), pp 144–148.
- Ho C-Y et al, “In vitro effects of preservatives in nasal sprays on human nasal epithelial cells”. Am J Rhinol, 2008, Vol22 (2), pp 125–129.
- “Questions and answers on benzalkonium chloride used as an excipient in medicinal products for human use”. PDF, EMA, 2013.
- “Air Pollution”. Web Page, WHO. (www.who.int/health-topics/ air-pollution, accessed April 2021.)
- Gänger S, Schindowski K, “Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico- Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa”. Pharmaceutics, 2018, Vol 10(3), p 116.
- “Nasal Flu Vaccine”. Web Page, Vaccine Knowledge Project (Oxford Vaccine Group). (vk.ovg.ox.ac.uk/vk/nasal-flu-vaccine, accessed April 2021.)

