Citation: Regard A, Chandra A, Farjas P, “Integrated Turnkey Solutions: Key to Successful Nasal Combination Product Development”. ONdrugDelivery, Issue 119 (Apr-May 2021), pp 25–28.
Alain Regard, Audrey Chandra and Pascale Farjas discuss the use of the nasal route for locally and systemic-acting drug treatments.
It’s that time of the year – the pollen season – and some of us might be experiencing what is commonly known as “hay fever”. One of the most common chronic conditions for which medical care – such as a nasal spray – is sought is rhinitis. Currently, approximately 10–30% of adults and 40% of children are affected.1 In fact, rhinitis can be induced by allergic stimuli, non-allergic triggers or both (mixed rhinitis). Allergic rhinitis only occurs in patients with a genetic predisposition to developing allergies.
“Drug administration via the nasal route avoids the hepatic first-pass effect which could be encountered when taking medications orally.”
Whilst the underlying mechanisms behind non-allergic rhinitis are quite variable and less well understood, nasal symptoms can be triggered by environmental irritants, such as smells and particulate materials, as well as changes in weather and barometric pressure. Some patients respond to emotional stress, hormonal changes and other unidentified stimuli.2
Allergic rhinitis is thus mostly seasonal – a hypersensitivity reaction is triggered when allergens are present. The symptoms of such a condition can often be relieved and controlled; a nasal spray containing topical-acting medications is normally prescribed for patients to treat the affected nasal cavity regions.
THE NASAL ROUTE USED FOR LOCAL AND SYSTEMIC-ACTING TREATMENT
Other than treating conditions locally, nasal delivery is also an attractive option to administer systemic therapies to target a range of conditions, from benign to serious. What’s more, the nasal route is non-invasive and does not require a healthcare professional’s intervention when patients can self-administer their remedy, to rapid effect. Additionally, it provides better bioavailability as drug administration via the nasal route avoids the hepatic first-pass effect which could be encountered when taking medications orally.
Taking into account these advantages, there is much to explore with the intranasal route. The anatomy of the nose allows for the administration of medications systemically or locally by targeting the optimal nasal region for drug deposition to eventually treat certain pathologies. To precisely address the exact part of the nasal cavity, the delivery device used within the drug combination product is a critical element and may vary depending on the therapeutic indications.
In Figure 1, for instance, allergic rhinitis and sinusitis are two of the pathologies which could be treated with topical medications. These are often relieved by nasal spray administration to reduce inflammation. More recently, there has been growing interest in delivering drugs via the nose for systemic conditions. This can be explained by the nasal turbinates occupying a large surface area of the nasal mucosa and being highly vascularised, which therefore is convenient for systemic delivery. As an alternative to conventional injections, certain vaccines could also be delivered via the nasal route as nasal associated lymphoid tissue (NALT) is connected to the lymphatic network and can induce a mucosal and systemic immune response.
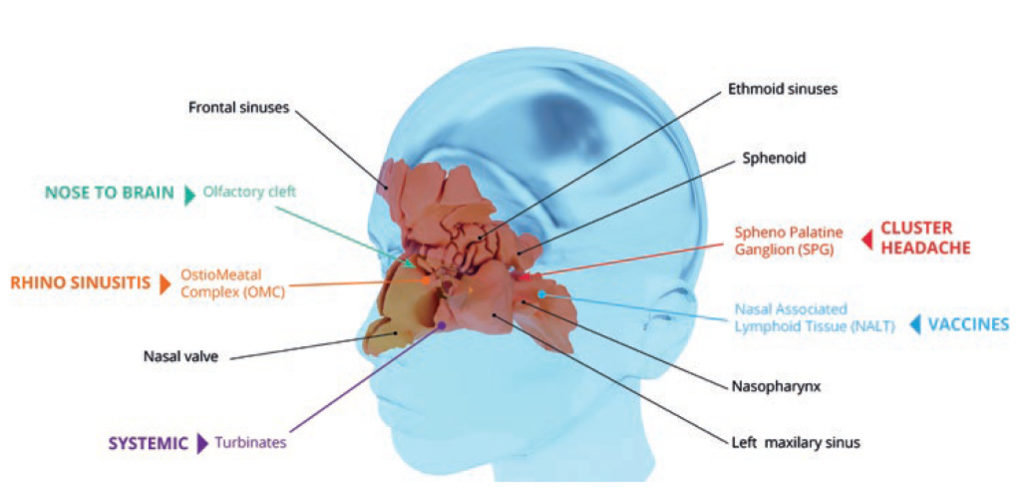
Figure 1: 3D rendering of nasal anatomy and its entry points for topical and systemic therapies to treat different conditions.
“Specific expertise and a holistic approach are crucial when developing a combination product for administration via the nasal route to ensure optimal region targeting and drug deposition efficacy.”
Therefore, specific expertise and a holistic approach are crucial when developing a combination product for administration via the nasal route to ensure optimal region targeting and drug deposition efficacy. Another aspect of intranasal delivery being explored is a nose-to-brain pathway that can be targeted through the olfactory region to treat central nervous system (CNS) conditions such as Alzheimer’s disease. In addition, cluster headache can be treated via the sphenopalatine ganglion (SPG). Cluster headache occurs in cyclical patterns, with cluster periods that may last from weeks to months (average 30-40 days) typically followed by remission periods. To block the pain, a numbing drug is applied to the SPG, an area which consists of a nerve bundle located deep inside the nose.
EXTENSIVE END-TO-END CAPABILITY OFFERINGS
Nemera has several decades of experience developing and manufacturing complex nasal devices – with a wide range of successful market references across the globe, both in regulated and low-regulated countries – to ultimately improve the quality of life of end users. The company has robust large-scale GMP manufacturing capabilities and produces millions of various nasal spray pumps every year. These devices are used to deliver drug formulations through the nose, optimised for drug efficacy and patient or care-giver use. Today, millions of patients rely on Nemera devices to treat their acute or chronic conditions, such as allergic rhinitis, sinusitis or nasal congestion.
“Delivering the drug product to the target site is challenging in the case of nasal delivery.”
“We put patients first” is Nemera’s principle. Its development team works actively to understand patients’ needs through discussions and tests with patients, individually or within groups, and round tables with key opinion leaders. Early-stage development concepts and insights go through user test studies to ensure a profound understanding of patients’ unmet needs. This is to optimise the intuitiveness and usability of the device, with the aim of reducing occurrences of misuse and optimising the performance of the device when handled by patients.
Delivering the drug product to the target site is challenging in the case of nasal delivery. Nemera continues to work on nasal delivery system concepts that target specific areas of the nasal cavity for optimum drug efficacy. To illustrate, the company uses in vitro testing on nasal casts which replicate human anatomy to predict the in vivo deposition. To support internal projects, Nemera has developed its own nasal cast, which can also support joint development efforts with customers towards a successful combination product.
Expertise in spray technology is also key to nasal drug delivery. Understanding the physics of the atomisation and achieving good control of the spray characteristics are two of the R&D pillars for nasal drug delivery. Nemera actively works on design processes and tools to support its spray development and reduce the number of design iterations required.
The company offers design services and customised development starting from a design brief, an idea and a concept. This design phase is finalised through a validated design which can be followed by stability and clinical samples, all the way to industrial volumes.
Furthermore, Nemera fosters a holistic approach in its partnerships with pharma companies, helping them navigate through their combination product development. In this way, not only does Nemera offer its expertise in robust device design and state-of-the-art manufacturing capacity, it also accompanies its customers throughout their development with its integrated front-end solutions. Thanks to its high end laboratory facilities, Nemera offers device-plus-formulation test services, as well as test method development for customers’ needs. In addition, the company’s regulatory experts are ready to help guide projects through a dynamic regulatory landscape.
In the case of establishing bioequivalence, following regulatory guidance is crucial. Thanks to its in vitro testing capabilities, Nemera can support specific generic projects with a complete set of tests that meet the regulatory requirements. The generated data is statistically analysed with respect to US and EU guidelines for customers’ eventual in vitro bioequivalence (IVBE) dossier registration filing.
Nemera also provides full understanding of the patient journey and recommends user-related activities to further optimise patients’ experience with a combination product. Thanks to Nemera’s extensive human factors capabilities, pharma companies can ensure their selected device, in combination with their drug, is appropriate, safe and effective for the target population. For example, Nemera’s support includes making instructions for use (IFU) adapted to specific target populations, as well as supporting human factors activities in alignment with a pharma company’s chosen regulatory path, including ANDA versus NDA.
Incorporating patients’ insights as early as possible to feed into product development is crucial. This is done by leveraging the patients’ journey throughout the drug delivery device R&D process to ensure that the device addresses the unmet needs of the end users. Through capabilities in human factors engineering, user experience design, engineering, lab services, statistical expertise and regulatory support, Nemera is uniquely positioned to offer all the support that customers require through an integrated device platform and service programme (Figure 2).
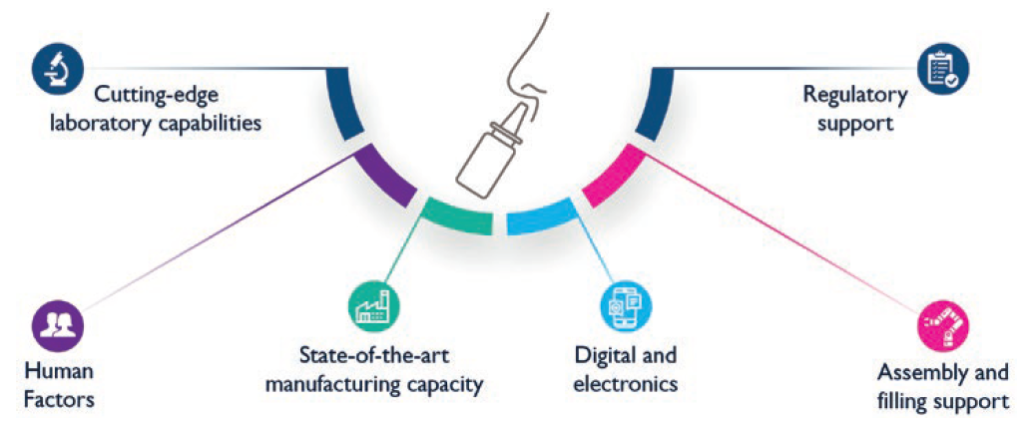
Figure 2: Nemera’s full turnkey solutions to support nasal combination product development.
“Nasal vaccination is seen as an attractive and complementary option to traditional injectable vaccines, especially in the covid-19 pandemic situation. There are more than 100 pipelines of nasal vaccines under development today.”
HOW NEMERA SEES THE FUTURE OF THE NASAL ROUTE
Although nasal sprays are mainly used for local therapeutic purposes (e.g. allergic rhinitis, colds, etc.) with conventional pump devices, new indications with systemic mode of action and associated delivery systems are emerging. Indeed, several rescue drugs have been recently launched using the nasal route, addressing opioid overdose, hypoglycaemia and seizures. This development can be easily explained by the attractiveness of the nasal route over injectable administration.
This change in mindset is a huge opportunity from a market perspective. However, to make the most of it, nasal drug products need to be as reliable and efficient as injectable ones. This opens the door to drug repurposing and product life-cycle management for the pharmaceutical industry, presenting a new administration route for existing drug products, either for acute needs, such as life-saving rescue medications, or for chronic diseases.
The development of such nasal sprays needs to be accompanied by a reliable device, with an accurate dose delivery and a specific deposition area in the nasal cavity, in order to achieve maximum therapeutic efficacy for the combination product.
To illustrate, nasal vaccination is seen as an attractive and complementary option to traditional injectable vaccines, especially in the covid-19 pandemic situation. There are more than 100 pipelines of nasal vaccines under development today, according to analytics data from Pharmacircle.
In addition, the RetroNose concept, with the principle of delivering drug product to the entirety of the upper airways, could also be advantageous in the fight against pneumoviruses and other pathogens. Last but not least, nose-to-brain drug delivery is another promising and challenging area, requiring the development of both a specific drug formulation and delivery system to reach the olfactory region in the upper part of the nasal cavity.
CONCLUSION
As a device developer and manufacturer, Nemera’s mission is to ensure proper drug delivery by depositing a precise amount of drug product in the right place, while fostering patient adherence via easy-to-use and intuitive devices. Nemera understands how important it is to continue exploring customised solutions for nasal delivery treatments to address unmet medical needs and to propose alternatives to the pharmaceutical industry, for both life-saving drugs and lifelong treatments.
The practice of embedding electronics into medical devices should provide further advantages and attractiveness for nasal delivery in the years to come. As such, Nemera has invested in R&D to develop various e-device concepts (e.g. Safe’n’Spray) and showcased the added value and technical features provided by electronics, which can be developed and customised according to customer needs.
In line with the growing interest in the nasal route for both local and systemic treatments, Nemera’s device platforms, coupled with its integrated end-to-end approach, enable it to support and bring drug/device combination solutions to patients.
REFERENCES
- Pawanker R et al, “White Book on Allergy: Update 2013”. World Allergy Organization, 2013.
- “In-Depth Review of Allergic Rhinitis”. World Allergy Organization, 2020.

