Citation: Halté C, “Interview: Cécile Halté. BD Medical – Pharmaceutical Systems”. ONdrugDelivery Magazine, Issue 99 (Aug 2019), pp 64-66.
Cécile Halté currently works as Senior Program Manager, Acute Care Segment, for BD Medical – Pharmaceutical Systems. Dr Halté’s focus areas are planning the product development roadmap over lifecycle of products for acute care and ensuring successful cross-functional execution of projects. She joined BD in 2012 and held various positions in project management, with increasing scope of responsibility. Dr Halté graduated from Ecole des Mines de Paris (France) and holds a PhD in nanobiotechnology from Université Joseph Fourrier (Grenoble, France).
In this interview, Dr Halté discusses validation studies of the BD Sterifill Advance™ plastic prefilled syringe, which is intended for use in the hospital setting by healthcare professionals either for manual bolus injections, or with infusion pumps.
“In a randomised and controlled study, it was shown that healthcare workers needed significantly more time for administering drugs to patients when preparing de novo in comparison with prefilled syringes in a stressful situation.”
Q Could you tell us why BD Sterifill Advance™ syringe has been developed, and describe it briefly?
A In a randomised and controlled study, and other studies, it was shown that healthcare workers needed significantly more time for administering drugs to patients when preparing de novo in comparison with prefilled syringes in a stressful situation.1-4
To address this issue, BD developed BD Sterifill Advance™, a common delivery system used in high stress, acute care settings, which is a plastic prefillable 50 mL syringe to be compatible with syringe infusion pumps. Later, BD Sterifill Advance™ was extended to smaller sizes in order to provide users with additional dose flexibility in use with pumps (20 mL sizes) or for manual bolus injections (5, 10, 20, 50 mL sizes).
BD Sterifill Advance™ 5, 10, 20 and 50 mL syringes are intended to be used by healthcare workers in hospitals, with infusion pump use (for 20 and 50 mL syringes) and manual use (for 5, 10, 20, 50 mL).
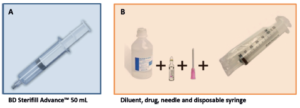
Figure 1: Materials required for infusions using BD Sterifill Advance™ 50 mL syringe (A) and disposable syringe (B).
Q What are the method and design elements of these validation studies?
A BD ran two studies to evaluate the expected impact of the new BD Sterifill Advance™ 50 mL prefillable syringe on infusion performance, healthcare workers’ daily practice and the consequences on patient and healthcare worker safety, comparing infusion with a syringe pump using either the BD Sterifill Advance™ 50 mL or a conventional system (drug in ampoule, diluent, disposable syringe).
A total of 300 infusions were performed by a biomedical technician with BD Sterifill Advance™ 50 mL (150) or a conventional syringe (150). Drug delivery performance was evaluated at four different flow rates (1, 5, 25 and 100 mL/h), using five commonly used syringe pumps.
To assess the usability of BD Sterifill Advance™ 50 mL 120 healthcare workers simulated 960 infusions, 480 with Sterifill Advance™ and 480 with a conventional syringe (Figure 1).
In the second study, 60 healthcare workers were enrolled and each of them performed eight infusions with BD Sterifill Advance™ 50 mL and two infusions with a conventional syringe in order to assess the usability of BD Sterifill Advance™ 50 mL with syringe pump.
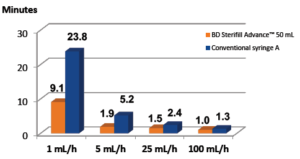
Figure 2: Time to reach 95% of targeted flow rate. Time in minutes to reach 95%
of targeted flow rates using BD Sterifill Advance™ 50 mL syringe and another
conventional 50 mL syringe with pump. N= 30 simulated infusions for both syringes.
BD conducted a third human factors validation study to assess the usability of BD Sterifill Advance™ for the manual infusion (5 and 20 mL formats) and in use with pumps (20 mL format).
For the human factors validation study for the manual injection, each of 15 enrolled healthcare workers performed 13 injections with BD Sterifill Advance™ syringes and two simulated injections with a conventional syringe.
Usability was evaluated through instructions for use (IFU) compliance. An observer recorded use errors (detection of operational difficulties). The steps successfully performed were counted, per simulation, per phase and overall, and errors were classified according to their risk class.
Q What were the objectives of the Human Factor Validation studies?
A The objectives of these studies were to demonstrate, using different syringe pumps, that drug delivery performance of BD Sterifill Advance™ 50 mL prefillable syringe is as good as the performance obtained with classic conventional syringe. Other objectives were to demonstrate, using human factors validation, that the Sterifill Advance™ 5, 10, 20 and 50 mL prefillable syringes are safe for patients and healthcare workers both for pump (20 and 50 mL) and manual (5, 10, 20 and 50 mL) use.
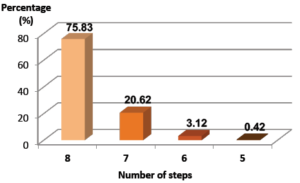
Figure 3: IFU compliance with BD Sterifill Advance™ 50 mL syringe per step.
Percentage of BD Sterifill Advance™ 50 mL simulations achieved with 1 to 8 IFU compliant steps. No IFU non-compliant steps between 1 and 4 steps. N = 480 simulated infusions
Q What were the results concerning BD Sterifill Advance™ prefillable syringe in combination with infusion pump?
A BD Sterifill Advance™ 50 mL syringe was found to be compatible with the tested syringe pumps at different flow rates (1, 5, 25 and 100 mL/h). Interestingly, BD Sterifill Advance™ had a significantly shorter T95% on average than the tested conventional syringe (Figure 2). By comparison with disposable syringes, the new Sterifill Advance™ 50 mL prefillable syringe reaches a satisfactory and equivalent drug delivery performance.
75.83% of the simulations were performed with BD Sterifill Advance™ by healthcare workers without any error (i.e. with eight IFU compliant steps) (Figure 3). At the steps scale, when using BD Sterifill Advance™, IFU compliance is fully satisfactory, with 96.48% of the steps having been correctly performed, and only 3.52% of the steps were performed with handling errors (IFU non-compliant) (Figure 4). Therefore, usability of the BD Sterifill Advance™ 50 mL syringe used in combination with infusion pump is validated.
From the last human factors study results, BD Sterifill Advance™ 20 mL syringe format has also been found safe and effective.
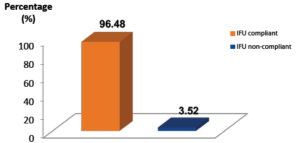
Figure 4: Overall IFU compliance with BD Sterifill Advance™ 50 mL syringe. Percentage of BD Sterifill Advance™ 50 mL simulations steps IFU compliant or non-compliant. N = 3840 steps.
Q Focusing on manual use, what results were obtained?
A In manual use, IFU compliance of BD Sterifill Advance™ 5, 10, 20 and 50 mL syringes was satisfactory (99.1% of steps correctly performed, on average) (Figure 5). The IFU were efficient based on results showing more than 99% success of the users that did not read the IFU. Overall, BD Sterifill Advance™ prefillable syringe was found to be intuitive (over 99% IFU compliance for all kinds of simulation at the first injection). Due to this high product intuitiveness, no perceptible learning effect was noted.
Therefore BD Sterifill Advance™ syringes have been found to be safe and effective when used by healthcare workers in hospitals for manual utilisation with no residual risk found for this application. It is important to note that these syringes may improve healthcare worker efficiency and effectively help save time for delivering treatment. Indeed, BD Sterifill Advance™ 5, 10 and 20 mL syringes have been found to be easy to use, useful and usable for experienced healthcare workers, with a faster preparation time compared with conventional syringes.
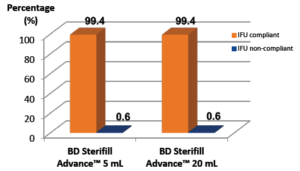
Figure 5: IFU compliance with BD Sterifill Advance™ 5 and 20 mL syringes.
Percentage of steps performed IFU compliant and IFU non-compliant using BD
Sterifill Advance™ 5 and 20 mL syringes for manual injections. N = 75 simulated
injections with respectively BD Sterifill Advance™ 5 and 20 mL syringes.
Figure 5: IFU compliance with BD Sterifill Advance™ 5 and 20 mL syringes. performed IFU compliant and IFU non-compliant using BD Sterifill Advancemanual injections. N = 75 simulated injections with respectively
REFERENCES
- Adapa R, et al, “Errors during the preparation of drug infusions: a randomized controlled trial”. Br J Anaesthesia, 2012, Vol 109(5), pp 729-734.
- Buerke B, et al, “Microbiologic contamination of automatic injectors at MDCT: experimental and clinical investigations”. AJR Am J Roentgenol, 2008, 191(6), pp W283-287.
- McDowell S, et al, “Where errors occur in the preparation and administration of intravenous medicines: a systematic review and Bayesian analysis”. Qual Saf Health Care, 2010, Vol 19(4), pp 341-345.
- Stucki C, et al, “Microbial contamination of syringes during preparation: the direct influence of environmental cleanliness and risk manipulations on end-product quality”. Am J Health Syst Pharm, 2009, Vol 66(22), pp 2032-2036.


