To Issue 131
Citation: “Interview: Jon Lenn & Jon Volmer, MedPharm”. ONdrugDelivery, Issue 131 (Apr 2022), pp 18–21.
Dr Lenn and Dr Volmer will be running a workshop at RDD 2022, Orlando, FL, US, on May 3rd, entitled “De-risking Pharmaceutical Nasal Development Programs”. They would be pleased to see you there.
“If you design a nasal delivery system properly, you can hit the olfactory bulb, which can result in rapid delivery of the drug directly to the CNS.”
Q To start with, can you give us a broad overview of what advantages the nasal route offers for drug delivery?
JV Nasal delivery is rapid, relatively painless and easy for the patient to administer, especially compared with injections. From a delivery perspective, the nose is highly vascularised, especially around the turbinate regions, so the nasal route is very attractive for systemic delivery. There’s also a moist surface for the formulation to cling to, so the drug can easily diffuse across the barrier and then get into the systemic circulation.
That’s only the beginning, however. One of the more exciting aspects of nasal delivery is that it can access the central nervous system (CNS). If you design a nasal delivery system properly, you can hit the olfactory bulb, which can result in rapid delivery of the drug directly to the CNS. So nasal delivery gives you a way to get drugs into the CNS that you simply can’t get any other way.
Thinking about practical applications, nasal delivery is well-suited for emergency medications, such as delivering naloxone to counteract opioid overdoses. You can use a nasal spray on someone who’s unconscious; you just spray the device in their nose while they’re breathing and the medication will take effect. Of course, the rapidity of nasal delivery is also a huge advantage in emergency situations; when a crisis occurs, you want the medication to kick in as quickly as possible – nasal delivery is perfect for that.
It’s also one of the few alternatives to injection for getting a large molecule into systemic circulation. Nasal delivery won’t work for all large molecules but it’s certainly viable for much larger molecules than, for example, topical administration via the skin. You can even get some proteins across the nasal epithelium.
Of course, there’s also interest in the nasal route from a vaccine perspective. With vaccines, you’ve got all the advantages I’ve outlined already – speed, little pain, systemic delivery and so on – coupled with the fact that the nose is often a primary route of infection for the diseases you’re vaccinating against, which is a major plus. Nasal vaccination is definitely a trend I think we’re going to see a lot more of.
JL To expand on that from the formulation side, nasal delivery has the advantage of not having to deal with a cornified barrier, instead you have active and passive transport that, in practice, simplifies the formulation approach. Compared with the skin, the obstacles you need to overcome to deliver a drug successfully via the nasal cavity are significantly less challenging. You do have to deal with mucociliary clearance, but that’s much easier to deal with than the protective barrier, or stratum corneum, of the skin.
“With MedCast, we can take the model apart into the specific functional regions for a specific modelled nose and then extract the deposited drug from each to see a relative ratio of drug in each functional region, which is what you really want to know from a drug delivery standpoint.”
Q Let’s discuss modelling. Briefly, why is it useful to model the nasal cavity when developing drugs for nasal administration?
JL When you’re looking at a drug-device combination product, you can examine and test the geometry and characteristics of the spray and the formulation as it comes out of the device. However, if that’s all the information you have, then you have to make some assumptions on where it is going to go when you spray it inside the nasal cavity. As such, you’re somewhat limited if you’re trying to deliver the formulation to a very specific location or if you want good data on how much drug is really being deposited at the target site.
That’s where physical modelling comes in. Conceptually, what we have done is create a physical 3D model where you can insert a device, spray the formulation and then break apart the main components of the cavity, extract the drug and quantify how much drug has been deposited in the different compartments, as well as potentially what may have gone into the lung. Therefore, modelling the nasal cavity allows formulators and device designers to access additional information that enhances their ability to optimise their product.
JV Modelling is particularly helpful for formulations that are trying to hit the olfactory bulb. If you’re targeting systemic delivery, it’s relatively easy to hit the turbinates, which is where you typically target delivery to get a drug into the systemic circulation. However, getting a drug through the nasal cavity to reach the olfactory bulb takes a specialised device. There’s also the inverse, where you’re targeting systemic delivery but need to make sure that the drug doesn’t hit the olfactory bulb at all – drugs where you do not want it going to the CNS. These two scenarios are where physical modelling is especially important because it offers that knowledge and increased awareness of exactly where the drug is being deposited in the nose.
Q Getting into the specifics, can you give our readers some detail on MedCast, MedPharm’s own nasal cavity model?
JV We started with CT scans of people’s heads and applied specialised modelling software to build digital 3D models, which we use as the basis of MedCast (Figure 1). We can then use 3D printing to make models out of various materials, which allows us to look at which materials are best suited for the chemistry of a specific drug-formulation combination. This variety of available materials gives us the ability to make sure that whatever material we’re using to make the MedCast in a specific instance will be able to bind the drug, hold onto it and then release it for analysis without modifying the device geometry.
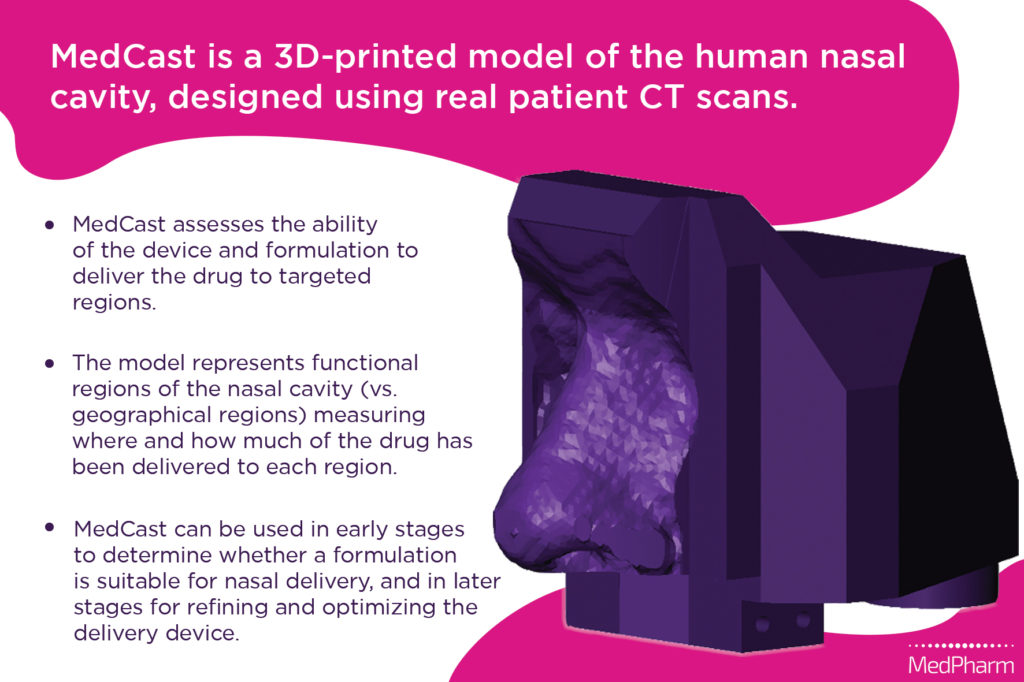
Figure 1: Digital render and summary of the MedCast model.
“You have further variation within each patient group and with MedCast, we’re aiming to get a representative sample across those differences.”
In terms of function, we can use a controlled airflow system in conjunction with the MedCast so that it models inhalation, exhalation or held breath, depending on how the nasal delivery device is intended to work. Then we administer the drug into the model and the drug distributes through it as it would in a patient’s nasal cavity. We can then disassemble the MedCast and quantify the drug deposition in the regions of interest.
There are other nasal models similar to MedCast but, generally, they divide the nose by geographic region – for example, upper, middle and lower. In contrast, MedCast divides into functional regions, which we identify based on anatomical cues from the specific CT scans that we use. This matters because it is the functional region that determines whether you’re going to get local, systemic or CNS delivery, or if the formulation is going to go down into the lung. With MedCast, we can take the model apart into the specific functional regions for a specific modelled nose and then extract the deposited drug from each to see a relative ratio of drug in each functional region, which is what you really want to know from a drug delivery standpoint. The ability to design MedCasts based on a cross-section of the population further reduces the risk once the device hits a real human population.
This plays a large part in why we’re using individual CT scans – MedCast is not just one model, it’s multiple models, all from different patient categories. For example, men and women are going to be different, children and adults are going to be different. Then you have further variation within each patient group and, with MedCast, we’re aiming to get a representative sample across those differences. So, depending on how detailed you want to be, we can use multiple different models based on the target patient demographic for a given drug.
JL An interesting note from the design process is that we’ve found that the model works best when we make the front part of the nose out of a pliable material. With the early prototypes, we quickly realised that, if you made the nostrils too hard, the model wouldn’t accept a device properly. Once we started making the front out of more pliable material, we were able to play around a little bit with the orientation of a device if needed. The end result being that you have the added advantage where, while not perfectly realistic, MedCast is close to what the front part of your nose would feel like.
“MedPharm has the advantage that we are able to do all of this in-house. By performing a broad sweep of development tasks co-ordinated under one umbrella, you reduce the risk of losing
information while it’s being transferred from one group to another.”
Q How does MedCast sit within MedPharm’s operations as a CDMO?
JL As a CDMO, we’re able to put an additional level of rigour into development, analysis and increasing throughput that wouldn’t necessarily be possible if innovation and modelling weren’t built into our processes. With MedPharm, a partner can come to us and we can help them get their API into a device and start testing it, and MedCast is a key part of that – it’s all in-house, which allows us to optimise our development programmes and really get the most out of them.
JV MedCast is useful in multiple ways for drug development. First off, you can use it right at the start of development to determine whether or not a formulation is suitable for nasal delivery in the first place – you don’t want to get to the stage where your formulation is in a nasal delivery device only to realise that it’s the wrong delivery route. Then, when you get a formulation that is suitable, MedCast is ideal for refining and optimising the device so that it delivers the formulation where you want in the quantity you want.
Q To round things out, let’s discuss MedPharm’s operations more broadly. Can you give us an overview of where MedPharm sits within the drug delivery sphere and what your core competencies are?
JL At a very high level, MedPharm’s core expertise is topical and transdermal. We’ve put all our effort into that specific niche so that we’re able to focus on really developing expertise that wouldn’t be possible if we diluted our attention across more delivery routes. To clarify, we define topical administration as delivering a compound locally to a tissue, and then transdermal delivery is interfacing with epithelium, aiming to deliver the drug into the systemic absorption. With these definitions, we’re somewhat agnostic when it comes to the epithelia in question – it can be the skin, the nose, the eyes or any other easily accessible mucosal epithelium.
This focus strongly influences how we approach everything, from formulation design to the biology itself. It also means that we’re able to develop very specific models for the routes of administration we excel in. Going back to MedCast as an example, we also have a reconstructed model of the nasal epithelium built from primary human nasal epithelial cells that helps to get a clearer picture of the biology. Coupled with practical data on deposition during delivery in the MedCast, we can help optimise that formulation and really de-risk a development project before it gets into the clinic. So, these are all preclinical and non-clinical assays that we highly recommend to make sure that you have the best formulation and device prior to undertaking expensive clinical trials.
JV MedPharm has the advantage that we are able to do all of this in-house. By performing a broad sweep of development tasks co-ordinated under one umbrella, you reduce the risk of losing information while it’s being transferred from one group to another. That can be a significant problem if, for example, you have one group working on the formulation, another on the device and yet another running tests with a nasal cavity model. So, while we’re working on a nasal formulation, in addition to doing performance testing with MedCast, we can also be looking at irritations, potential adverse effects in the epithelium, delivery through the nasal epithelium and, in some cases, drug effect assays.
JL In summary, developing that robust and rigorous package in order to enter the clinic is what we do best.

