To Issue 149
Citation: “Interview with Arnaud Guillet and Fanny Sellier of Biocorp.” ONdrugDelivery, Issue 149 (Jun 2023), pp 26–29.
In this exclusive interview with ONdrugDelivery, Arnaud Guillet and Fanny Sellier talk with Guy Furness about how Biocorp has become a key player in the field of connected drug delivery, discuss the current landscape for connectivity in drug delivery and update us on its two major parenteral products – Mallya and Injay – as well as looking forward to the launch of the company’s next generation of connected drug delivery solutions.
Note: This interview took place before the announcement on June 5th, 2023, that Novo Nordisk had entered exclusive negotiations to acquire a controlling stake in Biocorp from its main shareholder, BIO JAG, followed by a mandatory simplified tender offer for all remaining outstanding Biocorp shares.
Q Biocorp was one of the first companies to see the potential of connected drug delivery devices; can you describe how your prescient early move into the connectivity space gives the company a strong position as an established key player, while many other companies are just entering the space.

Figure 1: Mallya – Biocorp’s connected smart injector.
AG Entering into the connected drug delivery space was a key intention of our founder, who saw many unmet needs and issues in the field of pharma engineering and drug delivery – in particular, the lack of treatment management support for patients with chronic conditions, poor adherence to treatments among those patients and a lack of objective data and feedback for healthcare providers (HCPs). He was convinced that connected technology was relevant and mature enough to tackle these issues, and so he decided to structure Biocorp with the objective of developing this technology in mind.
In the very early days, around 2013, he decided to merge Biocorp’s historical device development and R&D capabilities with a company specialised in software developments. This gave Biocorp the necessary expertise – including mechanical, electronic, hardware, firmware and software – to develop its connected device programmes. I believe this is what makes Biocorp unique in this space because, for a relatively little company of only around 80 people, we have all this concentrated expertise inside. So, right from 2013, we were able to launch our first connected programme, DataPen, which was a motordriven pen injector featuring Bluetooth Low Energy connectivity. A couple of months after that, we launched the Mallya programme of smart injectors, which is currently one of Biocorp’s strongest assets (Figure 1).
Since those initial launches, we’ve worked on dozens of connected device programmes, mainly in the field of injectables. We’ve learned a lot from these programmes, including how to build a solution that is easy to implement for our partners and easy to use for patients. This means a design that doesn’t include too many functions or stimuli, and with an optimised form factor.
We’ve also improved our efficiency with respect to energy and resources, while also increasing our capacity for industrialising electronic devices. Furthermore, we’ve focused on the quality and regulatory departments, which are critical to getting products on the market. Thanks to these efforts, I believe we have become a reference point in the connectivity field for the drug delivery industry. We’ve achieved significant milestones, including the regulatory clearance of several drug delivery devices and the signing of major partnerships with pharma companies. We’re excited about launching with these partners and completing the product development journey.
When it comes to other companies potentially entering the connectivity field, I think that’s a good thing because we see that people need education and awareness around connected devices. More companies helping to build the awareness has the potential to be a real benefit to the field. There’s room for many players here – there are many different angles to tackle. We’re happy to see the community that is building around connectivity.
“We’ve achieved significant milestones, including the regulatory clearance of several drug delivery devices and the signing of major partnerships with pharma companies.”
Q One of the key questions occupying the connected drug delivery space at the moment is that of interoperability. What are your thoughts on how best to facilitate interoperability with apps and ensure that data flows are secure?
FS We cannot sell a device without apps, so for us it is key to have this interoperability with software partners. Of course, it is also very important to secure the communication between the device and the software. This is what Mallya brings – secure and safe transfer of data and the ability to handle various use cases that can happen with the device.
Interoperability is important for accelerating integration so that we reach the market as quickly as possible. To this end, we’ve developed an integration process and have been steadily improving it in order to shorten the integration period with software providers. This means more apps, and the more apps, the better, because we want to reach as many patients as possible – one app does not fit all, so we need to have many different integrations to cover the different specifics, such as geographies, type of patients, etc.
In order to simplify this, we are also currently working on developing a software development kit (SDK) to standardise the process. The SDK will help our software and pharma partners to simplify development and reduce development time and costs on their end by relieving a lot of the coding burden – they will only have to develop the user interface and user experience. Our goal is to simplify as much as possible by doing the work once and enabling everyone to benefit. We’re aiming to have the SDK ready by the end of this year.
AG In the context of a worldwide product launch with a pharma company, we’ve realised that a one-size-fits-all approach does not work. For example, some companies consider launching one single ecosystem together with their connected device but, in practice, this approach clearly doesn’t match up with differing local requirements. So, we’ve had to find a way to multiply the number of integrations in the fastest and most secure way possible, which, for us, is the Biocorp SDK.
“We’re excited about these launches because all patients dealing with a chronic illness, anyone who uses an injector pen, can get real benefit from a smart device like Mallya.”

Figure 2: SoloSmart
– a next-generation
Biocorp connected
device developed in
partnership with Sanofi.
Q The new generation of Mallya, Biocorp’s flagship connected device, is currently being launched. What’s improved with the latest version?
FS The big difference is that the first generation of Mallya had two pieces whereas the new generation is a one piece device. This makes it much easier for patients to attach it to their injector pen. Additionally, the new generation does not need to be recalibrated in the way previous generations did. Overall, the new generation has been designed to be easier to use and more patient friendly. Also, because the new generation is a single-piece device, it is a little bit less costly as well compared with its predecessors. It’s a win-win for everyone.
With respect to launching the new generation of Mallya, our aim is to launch in most worldwide markets this year. For example, the new generation will be launched in Asia and Europe this year for insulin. You may have seen that the new Mallya has already launched in Japan, in March of this year. Outside of Mallya, we also have some devices like SoloSmart (Figure 2), which we designed for Sanofi, that we’re aiming to launch soon in different countries in Asia and in Europe.
Our launches this year are primarily for insulin. However, we also have upcoming launches for growth hormone treatments, which also will be launched in 2023, but I cannot disclose the name of the company we’ve partnered with or the product name just yet. What I can tell you is that some Biocorp devices for growth hormones will be launched this year in several countries worldwide.
We’re excited about these launches because all patients dealing with a chronic illness, anyone who uses an injector pen, can get meaningful benefit from a smart device like Mallya. For example, with insulin, it’s great to have Mallya to keep track of the details of each injection to make it easier for a patient to look at their injection history and how their treatment is going. It also facilitates good communication with HCPs, giving them objective data to discuss with their patients and much better evidence of how the treatment is going.
This is true for much more than just insulin and diabetes, of course. For example, fertility is another field we’re working in, and there Mallya can provide patients with real reassurance. It allows patients to follow along with and feel more comfortable about their treatment and lets their HCP see that they are taking their treatment seriously. It’s very important with fertility treatments to administer the drugs at certain times and dates, so it’s important to use the best available technology to track these injections.
Q Please could you explain why digital ecosystems are so important in diabetes and how Biocorp is driving the technology forward in this area?
FS With diabetes, there is a very high treatment management burden on patients, so they need the fullest support available. For example, it’s better for patients to have their glucose monitoring in the same app as their insulin tracking to make it as easy as possible for them to keep on top of everything. This is a key driver behind our partnership with Health2Sync (Taipei City, Taiwan), as part of which we’ve signed a partnership with them for Mallya’s launch in Japan.
A next step to take in this is developing semi-loop systems. So, on top of having glucose monitoring and insulin tracking, we can also include an insulin titration algorithm that can calculate the amount of insulin that the patient needs to inject. In this area, we’ve signed a partnership with Diabeloop (Grenoble, France) to take one step further into this system.
A key benefit of using connected technology with diabetes is that it makes it much, much easier to conceptualise the insulin data. By doing so, it’s possible to maximise the value of the treatment in a simple, safe and accurate way, and of course accuracy is incredibly important in diabetes. A good software partner can also put the data into the context of the patient’s daily life, such as their glucose level, activity level, carbs intake and a whole host of things like that. Putting all of this information together is key for the patient, and that’s what digital ecosystems excel at.
“Injay is a very simple and very cost-effective way to monitor
the adherence of medicines delivered with prefilled syringes.”
Q Considering the current interest in artificial intelligence (AI), is Biocorp investigating the use of AI in the context of connected drug delivery?
AG Yes, it’s certainly something that we’re exploring. For diabetes, I think there are a numerous good algorithms in the AI area being developed by software companies, so our current approach is to partner with them and make use of their expertise. For now, Biocorp’s key strength is collecting insulin data in a very safe, robust and accurate way – that’s the piece of the puzzle we want to provide. If we can do that, then the larger digital ecosystem will be able to build on it and maximise the benefit for patients. We’re currently considering taking on a bigger role in the field and perhaps developing proprietary software in the future, which could absolutely include AI-driven capabilities for recommendations and advice for patients to bring about more positive clinical outcomes.
There are two real and very exciting developments that will apply to drug delivery in the future: AI and behavioural science. The potential combination of these two trends is very interesting, because AI is based on using and analysing the data you acquire to better understand the condition of a patient based on their specific clinical conditions, whereas behavioural science is based on how a specific individual thinks and acts in both broad and specific terms, which is key to tackling low adherence – it’s not only about your condition, it’s about who you are, your personal belief regarding your disease and the way you behave as a person. If we’re able to provide better for patients based on who they are, we might see some real improvements in terms of adherence.
I think for diabetes, the digital ecosystem is mainly focused on glucose and insulin data collection – that’s the most critical part. However, outside of diabetes, in chronic conditions where there are less frequent injections and less frequent requirements for patients, the focus can shift to finding the right driver to keep patients taking their medication in a consistent way and seeing better health outcomes. I think this is somewhere behavioural science could really play a big role.
Q Biocorp’s other major connected add-on in the parenteral sector is Injay, which is compatible with various sizes of conventional prefilled syringes (PFSs). Please could you give an overview of Injay’s features and benefits, as well as its utility for the delivery of biosimilars?
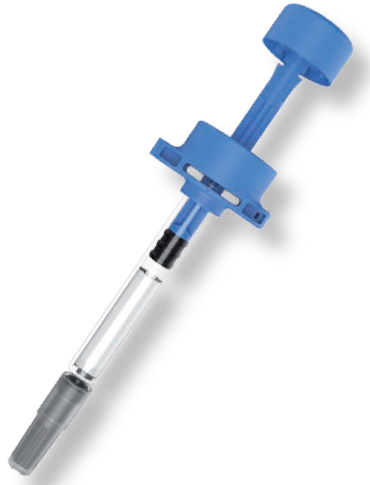
Figure 3: Injay – Biocorp’s connected PFS add-on solution.
AG Injay is a smart solution to track injection data on PFSs, composed of a near-field communication chip, the main purpose of which is to recall product information, and an activator to confirm complete injections. These components are directly installed by the pharma company onto the syringe, which is then delivered and ready to use by patients or HCPs. Once the PFS is used, the chip can be scanned to retrieve product information and confirm the complete injection together with a timestamp.
As such, Injay is a very simple and very cost-effective way to monitor the adherence of medicines delivered with PFSs (Figure 3). Additionally, Injay has limited environmental impact because there are no active electronic components installed on the syringe, making it freely disposable. Biocorp has developed one version that is applicable to naked PFSs in various sizes, including 0.5 and 1 mL – both long and short – and 2.25 mL. Also, we’ve partnered with BD to develop a specific version that is compatible with the BD UltraSafe device.
A key use case for Injay is for monitoring drug delivery in the context of clinical trials. Here, the primary benefit is the ability for the study operators to monitor patients’ adherence. This is critical, because we’ve found that between 30% and 50% of patients are non-adherent in clinical trials, which is often overlooked because it tends to be assumed that, if you’re participating in a clinical trial, you’ll be adherent – but that’s not always true. This, of course, then has a significant impact the results of the trial. This is where Injay comes in, because it enables the detection of non-adherence, the trial operators can act on that information and intervene with non-adherent patients, saving time and guaranteeing the quality of trial outcomes. It also facilitates efficient and accurate automatic data collection, which increases the quality of the data over traditional patient self-reporting by eliminating human error, as well as enabling hybrid and decentralised trials, which are currently increasing in number.
The other primary use case is with commercial drugs. In this instance, we’re talking about very expensive biologics, often in fields such as rheumatoid arthritis, dermatotic arthritis, psoriasis and multiple sclerosis. In these areas, a patient will have weekly injections, so Injay is a tool that can be used in combination with patient support programmes to keep a record of injection data. It can also provide HCPs with objective data on drug intake and the patient engagement – whether they are taking their drug or not. As well as that, it can provide payers with proof of actual drug usage in a real-life setting, which can enable value-based assessments; this is more a consideration for the long term, but a shift towards a value-based model is going to happen at some point, and Injay is able to facilitate that.
“It tends to be assumed that, if you’re participating in a clinical trial, you’ll be adherent – but that’s not always true.”
Q Looking at the big picture for the large-scale adoption of Biocorp’s connected drug delivery devices, can you map out some of the key milestones that you’ve already achieved and those you’re still aiming to achieve? Additionally, do you think that a clear regulatory pathway is a prerequisite to widespread industry and patient acceptance?
FS We have achieved US FDA approval for the first generation of Mallya, which was finalised late in 2022. This was a huge milestone for us and something on which we’ve been working very hard. We were proud to get this 510(k) for Mallya. We have also secured the CE mark for most of our new-generation products, including the SoloSmart. We have received further certifications from other jurisdictions around the world, mostly in Latin America and Asia.
What’s coming next is the internationalisation of all of our new generation products, starting with the new Mallya platform in December 2023. We’re currently in the ramping-up phase, launching all these devices onto the market in different countries. As part of this, we and our customers are also doing some real-world evidence studies as well. That’s the challenge we’re taking on in the immediate future. Looking further ahead, we’re continuously building on our clinical evidence and deploying market access strategies based on the specifics of each country to get reimbursement.
AG One way to think about it is that it’s like different stages of a rocket. The first stage is the technical development, which we’ve achieved successfully. Next is the regulatory stage, which was quite difficult, but we’ve now achieved that one as well. The third stage will be the pinnacle, building the clinical evidence and opening new doors for reimbursement. I think we are in the middle of this phase, building these networks together with doctors, building clinical evidence and creating awareness of the benefits of connected drug delivery. It’s an exciting moment for the company.

