Citation: Bertollo N, Rock A, O’Callaghan S, “It’s Time to Consider Intradermal Delivery”. ONdrugDelivery, Issue 166 (Oct 2024), pp 42–46.
Nicky Bertollo, Adam Rock and Saidhbh O’Callaghan discuss the progress Pharma Latch’s intradermal drug delivery platform is making towards realising the potential the intradermal route offers to drug developers, especially for vaccines.
Intradermal (ID) delivery has long held the potential to be an attractive route of administration across a range of drug delivery applications. However, due to the limitations of previous and current ID delivery technologies, this potential has never been realised. However, the Pharma Latch platform overcomes many of the obstacles that have previously been encountered in the pursuit of a successful ID delivery system, allowing ID administration to be considered a truly realistic route of administration for the first time, with a host of benefits across a range of applications.
In Pharma Latch’s previous article in ONdrugDelivery ,1 the company outlined the potential benefits of ID delivery across various drug types, including:
- For vaccines:
– Functional improvements in the durability, strength and type of immune response following vaccination
– Similar immune responses with fractional dosing (i.e. dose sparing). - For biologics:
– Improved pharmacokinetic profiles (increased bioavailability, faster onset of action, increased Cmax/Tmax, etc)
– Reduced needle phobia from shallow needle penetration aiding compliance - Targeted (versus systemic) delivery, reducing adverse events and improving the therapeutic window.
While many of these benefits are well studied and understood, the lack of simple, reliable and practical delivery methods and devices has meant that the ID route is rarely used in clinical practice.
For example, the Mantoux technique – a healthcare professional (HCP) inserting a needle into the skin at an angle, first described more than 100 years ago – remains the clinical gold standard for performing ID injections. This procedure is technically challenging to perform, as the approach angle and depth of needle penetration are user-dependent and therefore subject to a high degree of variability. In fact, it has been demonstrated that up to 70% of ID injections administered using this technique are delivered to the incorrect depth.2
Subsequently, a number of drug delivery technologies have been developed to improve the ease of ID delivery, such as microarray patches, jet injectors and other microneedle systems, however, these have achieved limited clinical and commercial success. Often, they struggle in some or all of the following areas:
- Difficulty in administering to the correct depth in the skin
- Regulatory concerns over consistent dose administration
- Lack of confidence for the administering HCP
- Typically very low volume (e.g. 100 μL) and viscosity capabilities (primarily aqueous-based)
- Difficulties around device manufacturing and scalability
- Reformulation requirements (for patches in particular).
For these reasons, the ID route has rarely been considered a realistic or attractive option for the pharmaceutical industry, as it can introduce unnecessary risk,a lack of confidence in the ability of devices to reliably deliver ID doses in full and added complexity, time and cost to development programmes. Additionally, many available technologies only offer a single device solution, with limited flexibility with regard to changes in drug dose or formulation and limited use cases, such as being suitable for administration by an HCP only.
Pharma Latch’s platform was developed with these shortcomings in mind. The company focused on the development of innovative device solutions that work with the needs of the pharmaceutical industry and patients, rather than adding complexity to them.
“The Pharma Latch platform was developed to overcome the biomechanical properties of the skin, which naturally resist needle insertion, and are a significant contributor to the inconsistency of previous ID injection technologies.”
THE PHARMA LATCH PLATFORM
The Pharma Latch platform is based on opposing arrays of angled hypodermic needles. As described in detail in a previous issue of ONdrugDelivery,1 the Pharma Latch platform was developed to overcome the biomechanical properties of the skin, which naturally resists needle insertion, and are a significant contributor to the inconsistency of previous ID injection technologies.
As a result, the Pharma Latch platform enables precise and repeatable ID injections, every time. Furthermore, the combination of a unique fluid pathway and favourable manipulation of the biomechanical properties of the skin allows the Pharma Latch platform to inject high-volume and high-viscosity formulations (up to 3 mL and 60 cP), increasing the clinical applications of ID delivery beyond what was previously thought possible. Pharma Latch’s novel injection platform also facilitates the development of a pipeline of future self injection and wearable combination products (currently under development) that can address the complex delivery requirements of therapeutics for the management of chronic diseases.
In summary, the Pharma Latch platform is a disruptive drug delivery platform that:
- Overcomes the technical challenges of ID delivery, allowing the ID route to be a realistic option for the pharma industry
- Enables new applications and use cases for ID delivery to be realised due to superior volume and viscosity capabilities
- Provides a platform solution with multiple device offerings capable of serving a wide array of drugs and use cases.

Figure 1: Overview of PLH, which is being developed as a single-use, sterile-packed, HCP-administered medical
device. The PLH is compatible with both Luer lock and slip syringes, and exhibits arrays of angled 31G hypodermic needles at its distal end for delivery of the injectate into the dermal layer.
PHARMA LATCH HOLLOW
The first product offering built off the Pharma Latch platform – the Pharma Latch Hollow (PLH) – is in development as an HCP-administered, sterile-packed, single use disposable medical device (Class II medical device, FDA 510(k) pathway) and is in late-stage manufacturing in the US (Figure 1). The PLH is available for preclinical work now (in small or large animals) and for clinical evaluation from 2025.
The PLH introduces a new and improved device-based solution for injecting liquid formulations into the skin, achieving this in a number of different ways:
- PLH dramatically simplifies the procedure for the user and removes the variability associated with traditional techniques by having device geometry (and not the user) govern needle insertion depth (Figure 2).
- Through a combination of favourable conditions that are established in the skin and inherently low internal fluidic resistance, PLH provides ease of injection and can work with volumes up to 3 mL and viscosities up to 60 cP, which were previously only achievable with intramuscular (IM) and subcutaneous (SC) administration.
- PLH ensures full dose delivery consistently and repeatedly in all patients, thereby satisfying clinicians and regulators alike.
- PLH can be used immediately with any drug formulation and dose without the requirement for reformulation and complex in vitro assessment.
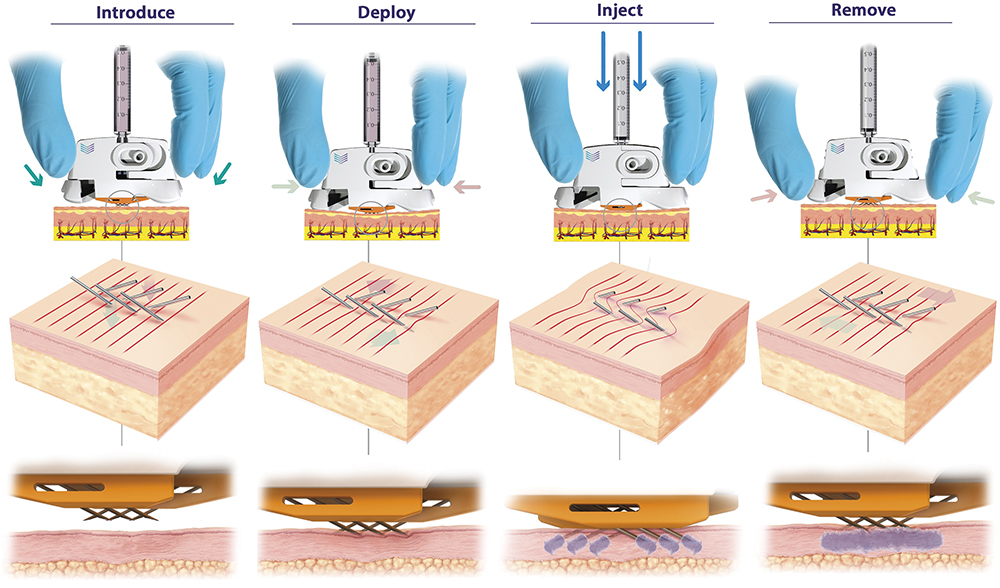
Figure 2: PLH operation (left to right). Device is placed over the injection site. The needles are then deployed using a simple “clicking” action, which establishes a slight stretching of the skin, preventing tenting and compression and enabling full penetration of the hypodermic needles to a predetermined depth (820 μm). Injection can be performed with minimal resistance and with superior diffusion into uncompressed tissue. The PLH can then be removed from the skin, using simple squeezing of the release tabs, and disposed of.
INTRADERMAL APPLICATIONS
PLH has been developed to serve a wide range of use cases, where ID delivery is increasingly being recognised as an enabling technology as well as a point of differentiation. Cancer vaccines (CVs) are one such area and have witnessed tremendous growth and investment in recent years, with approximately 360 active clinical trials.3 Although CVs have faced significant challenges in the past, advances in sequencing technology enabling the identification of new target antigens, successful combination therapy with checkpoint inhibitors and emerging vaccine technologies, such as messenger RNA (mRNA), are fuelling a renewed interest in this segment.
“The ID route is continuing to gain traction in the CV space, as evidenced by approximately one-third of current CVs in clinical trials being delivered intradermally.”
CVs are therapeutic vaccines, requiring strong and specific immune responses – typically over multiple doses per course, in order to be effective against the target disease. As such, there is a concerted effort to optimise all aspects of current CV design, including route of administration. There is a strong scientific rationale for administering CVs intradermally, as this approach takes advantage of the high density of antigen-presenting cells in the skin, such as dendritic cells, whose activation is critical for eliciting the desired immune response. The ID route is continuing to gain traction in the CV space, as evidenced by approximately one-third of current CVs in clinical trials being delivered intradermally.3
Similarly, other more traditional vaccine applications can also derive significant benefits from ID delivery, including dose-sparing – the ability to generate similar immune responses with a fraction of the dose. This dose-sparing effect has been demonstrated with fractional doses of one-fifth of the standard IM or SC dose, or even less, across numerous vaccine platforms, including mRNA, peptide, viral vector and live attenuated viruses. The ability to dose-spare has numerous implications and benefits for vaccines, including reducing systemic adverse events, reducing costs and, importantly, increasing critical supplies of vaccines, which is especially important for rapidly responding to disease outbreaks. In fact, in 2022, in response to an outbreak of monkeypox (Mpox), the US FDA authorised the ID delivery of the JYNNEOS vaccine (Bavarian Nordic, Hellerup, Denmark) at one-fifth of the SC dose, enabling rapid roll-out of the vaccine.
Biologic drugs, such as peptides and monoclonal antibodies (mAbs), continue to represent a significant proportion of new drug approvals, with an increasing proportion now being delivered subcutaneously. While this transition has been successful, a number of key challenges remain, including concerns around patient compliance, variable pharmacokinetics and suboptimal efficacy. ID delivery of biologics has numerous well-established benefits in this regard. For one, while SC administration allows for self-administration, this places a high degree of burden on the patient to comfortably perform a correct injection. In addition to needle-phobia, which is a well-established phenomenon, concerns around uptake and compliance must be addressed. ID delivery offers a potential solution in this regard, as Pharma Latch’s microneedles reach a depth of 820 μm in the dermal layer, which can help reduce any anxiety surrounding needles and may improve compliance.
“ID delivery has demonstrated a clear benefit across numerous high-value biologic therapies, including checkpoint inhibitors, insulin, GLP-1s and other mAbs.”
There is also increasing evidence to suggest that the ID route has therapeutic and pharmacokinetic benefits compared with SC and IM delivery. These benefits are thought to arise from the improved access to the lymphatic network following ID administration, which enables rapid transport to the systemic circulation, especially for larger molecules, as well as a potential secondary site of action for some immunotherapies. ID delivery has demonstrated a clear benefit across numerous high-value biologic therapies, including checkpoint inhibitors, insulin, glucagon-like peptide 1 (GLP-1) and other mAbs.4–9
WORKING WITH THE PHARMA LATCH PLATFORM
The ability of the Pharma Latch platform to deliver higher volumes and viscosities than previously possible (up to 3 mL and 60 cP) via the ID route opens up a wide range of additional applications. The platform has a range of iterations (Figure 3):
- Preclinical Study Devices: Available now – Pharma Latch has developed a range of specific versions of PLH to work in a variety of both small and large animal models. This is facilitating early, accurate evaluation of the ID route of administration in a host of development programmes.
- HCP-Administration: Available now – Pharma Latch’s PLH device as outlined above is a medical device available for preclinical and clinical work with approval anticipated in H1 2025.
- Self-Administration: Application dependent – Pharma Latch’s platform approach retains the same fluid pathway and manner of skin interaction, allowing users to work with PLH and migrate to a range of self-injector versions of the platform specific to customer requirements.
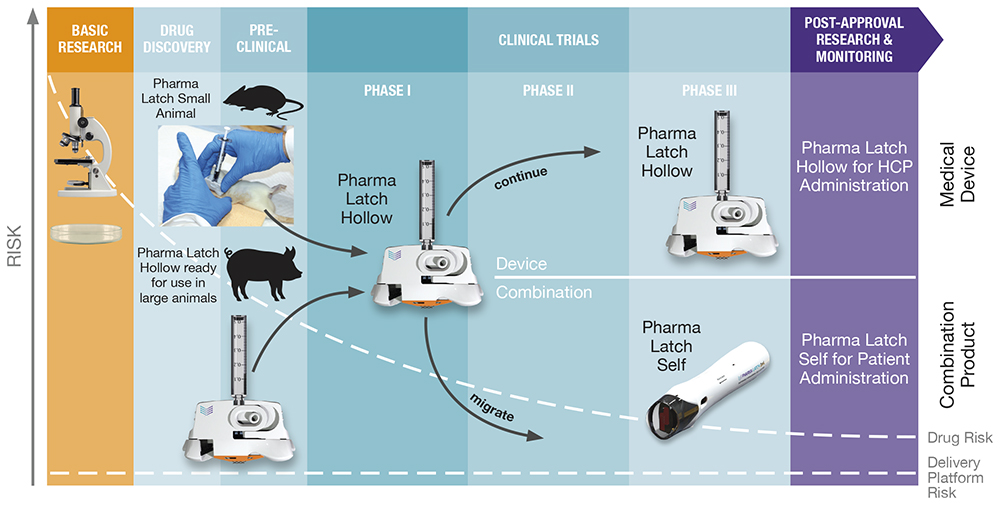
Figure 3: The Pharma Latch platform has been incorporated into a series of devices that can be used across the R&D timeline, from early preclinical through to market approval, and a variety of use cases can also support a number of different use cases, including HCP-administered devices or self-administered combination products.
SUMMARY
An effective delivery strategy is fundamental to bringing innovative therapies to market. As drug modalities have evolved from small, simple molecules to complex biologics, unique delivery challenges have emerged, and drug developers are increasingly searching for innovative delivery solutions. ID delivery has long been considered an attractive solution in this regard and, for the first time, is now a reality with the Pharma Latch platform.
REFERENCES
- Bertollo N, Rock A, Byrne R, “How Do You Perceive Intradermal Delivery? Think again…”. ONdrugDelivery, Issue 160 (May 2024), pp 26–30.
- Micheels P, Goodman L, “Injection Depth in Intradermal Therapy: Update and Correction of Published Data”. J Drugs Dermatol, 2018, Vol 17(1) pp 88–96.
- Janes ME et al, “Cancer vaccines in the clinic”. Bioeng Transl Med, 2023, Vol 9(1), article e10588.
- Rini CJ et al, “Intradermal insulin infusion achieves faster insulin action than subcutaneous infusion for 3-day wear”. Drug Deliv Transl Res, 2015, Vol 5(4), pp 332–345.
- Ranamukha S et al, “Intradermal Administration Improves the Kinetics of Faster-Acting Fiasp® Insulin”. Diabetes, 2018, Vol 67(Suppl 1), article 985-P.
- Jacobse J et al, “Comprehensive evaluation of microneedle-based intradermal adalimumab delivery vs. subcutaneous administration: results of a randomized controlled clinical trial”. Br J Clin Pharmacol, 2021, Vol 87(8), pp 3162–3176.
- Hoeijmakers LL et al, “The MARIANE-trial: Multicenter phase 1b/2 trial testing safety and efficacy of neoadjuvant intradermal ipilimumab and nivolumab in high-risk stage II melanoma”. J Clin Oncol, 2024, Vol 42(16_ suppl), article TPS9615.
- Tanaka R et al, “Efficient drug delivery to lymph nodes by intradermal administration and enhancement of anti-tumor effects of immune checkpoint inhibitors”. Cancer Treat Res Commun, 2023, Vol 36, article 100740.
- Van Pul KM et al, “Local delivery of low-dose anti-CTLA-4 to the melanoma lymphatic basin leads to systemic Treg reduction and effector T cell activation”. Sci Immunol, 2022, Vol 7(73), article eabn8097.

