To Issue 149
Citation: Flaherty-Earp F, “Lessons from Marketed Medical Devices to Enhance Connected Drug Delivery.” ONdrugDelivery, Issue 149 (Jun 2023), pp 16–18.
Fallyn Flaherty-Earp looks at what medical device manufacturers can learn from connected devices already on the market and discusses the opportunities electronic inks can provide for the development of innovative therapies for the treatment of chronic diseases.
It is a testament to the seismic growth in interest surrounding connected drug delivery that the column inches dedicated to the topic continue to grow exponentially, too.
One particular aspect of that growth is attributable to the development of wearable devices, often referred to as on-body delivery systems (OBDSs). OBDS platforms can now deliver higher volumes of medicines that would otherwise need to be administered intravenously at a clinic under the supervision of a healthcare professional (HCP). This development follows an increasing demand for treatment away from clinical settings, enabling patients to realise greater choice and control over their disease management.
It is in this context that drug delivery partners can learn a great deal from medical device innovations, where there is already an established portfolio of customised, connected solutions available for different patient groups, across each key stage of the human lifecycle – from prenatal monitoring to elderly and palliative care.
“With better quality data and insight comes greater personalisation in treatment regimens enabling individualised sensors and tools shaped to the needs of the patient.”
MARKET TRENDS IN MONITORING AND PERSONALISATION
As the OBDS market is expected to grow, so too is the remote patient monitoring market, which is set to reach US$175 billion (£141 billion) by 2027, growing at a compound annual growth rate (CAGR) of 26.7%.1 The narrative behind this trend is the same as in OBDS – HCPs and patients continue to prioritise at-home care over visits to healthcare facilities in the post-pandemic era. And that is not the only comparison between trends in medical devices and drug delivery. There is growing demand for artificial intelligence (AI) to be incorporated into medical wearable devices to help with data analysis, interpretation and delivering insight that can help with diagnosis and treatment. This market is projected to expand at a CAGR of 29.8% from 2023 to 2030.2
With better quality data and insight comes greater personalisation in treatment regimens enabling individualised sensors and tools shaped to the needs of the patient. This personalisation includes integration with other devices, such as smartphones and smartwatches, that can provide patients with a more integrated and convenient healthcare experience.
The final trend we are witnessing is the continued development in non-invasive monitoring, such as heart rate and blood oxygen level monitoring, enabling patients to manage their own assessments and treatment more intuitively, simply and with less HCP intervention.
As everyone in the drug delivery ecosystem would recognise, improved patient outcomes, personalisation and at-home care are all key considerations for the development of next-generation devices.
OPPORTUNITIES WITH ELECTRONIC INKS FOR PRINTED SENSORS
To maximise the opportunities presented by these emerging trends, several enabling technologies are required – not least the use of electronic inks or conductive inks. These inks are used increasingly in the production of wearable medical devices due to their ability to create flexible and conformable electronic components (Figure 1).
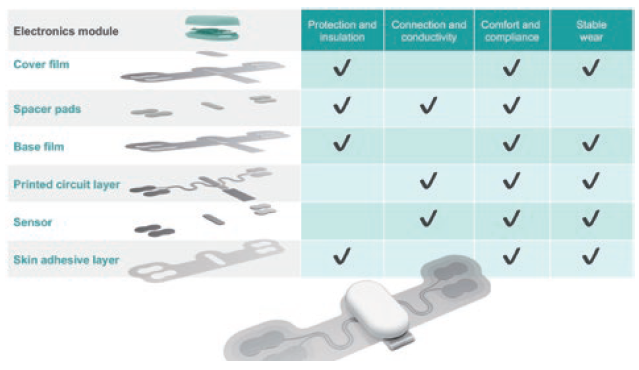
Figure 1: Anatomy of a wearable sensor – sensor technology for dry electrodes provides highly effective recording of biosignals when integrated into skin patches or textile devices.
Electronic inks, such as Micromax™ Biomedical Sensor Inks from Celanese, are used to print sensors onto wearable devices that can monitor various physiological parameters, such as heart rate, blood pressure and oxygen saturation. These sensors are typically made of conductive materials such as silver, silver/silver chloride or carbon, and are printed onto a flexible substrate, such as polymer film. The sensors can then be integrated into skin patches, garments, wristbands or other wearable devices, allowing for continuous monitoring of a patient’s vital signs.
Electronic inks are often used in the production of electrodes for electroencephalography and electrocardiography monitoring. These electrodes are typically applied to the skin using a conductive gel or adhesive, which helps to ensure a stable electrical connection between the skin and the electrode. These gels and adhesives are also designed to be skin-friendly, minimising patient irritation or discomfort.
The make-up of the electronic inks is critical to the delivery of medications and accuracy in patient monitoring. Split into three main material types for medical purposes – conductive, dielectric and resistive – these inks are applied to achieve a range of functionalities.
Conductive materials are used to create conductive tracks, pathways and component terminations, including inner electrodes. Dielectric materials provide insulating (nonconductive) layers. They may be crossovers or multilayers with connections between layers. Resistive inks are partially conductive compositions that reduce electrical current. The quality of the ingredients and manufacturing of the electronic inks are crucial to how well the inks will transmit a signal. Electronic inks are typically developed for specific healthcare applications to ensure that they meet the requirements of use, including resistivity, stretchability, washability, chemical resistance, etc.
One therapeutic area where electronic inks show real potential for effective drug delivery is in the treatment of cancer. Electronic inks can be used to deliver chemotherapy drugs directly to cancer cells, minimising the damage to healthy cells through electrochemical gradients and targeted release triggered by electrical signals. Another potential application is in the treatment of neurological disorders, such as Parkinson’s disease and epilepsy, where electronic inks can be used to deliver drugs to specific areas of the brain, allowing for more precise treatment of conditions.
Electronic inks are being explored as a viable combination drug delivery/diagnostic option for the treatment of chronic diseases, such as diabetes. They can be used to create printable biosensors that monitor glucose levels in real time – information that can then be used to trigger the release of insulin in response to changes in glucose levels (Figure 2). Similarly, electronic inks can be used to create printable sensors that can detect the presence of bacteria that then trigger the release of antibiotics in response to bacterial growth.

Figure 2: Highly conductive inks, dielectric and fine-line printing solutions enable new possibilities for eHealth product designers, including multi-layer stacking and miniaturisation.
“Ergonomically, a smart patch should be so comfortable that the patient can forget they are wearing it in normal everyday life.”
WEARABILITY AND RELIABILITY: A CASE STUDY
The following case study reviews the development of an adhesive patch designed to deliver wearability and long-term comfort for patients, while reliably recording electrical signals for vital signs and physiological parameters.
The ability to effectively monitor an individual’s health conditions – with electrical body signals, for example – and then deliver clinical-grade data for analysis without delay is revolutionising healthcare. To support fast market adoption of wearables at the original equipment manufacturer and patient levels, several design challenges must be overcome.
One challenge is to achieve long-term, accurate monitoring of biosignals with a device attached for a few days – or up to as many as 14 days. Currently available technology is limited by the battery lifetime, the electrode performance, and the device wearability on skin when exposed to sweat, humidity and bathing water. Also, skin contact patches potentially need to adapt to several skin types, based on age, gender and culture, and will require versatile patch adhesives.
Another significant design challenge is to improve the patient experience with a patch that is easily applied, comfortable and non-irritating, stays fixed in place for days during regular living activities and can be removed painlessly without causing trauma at the end of the procedure. The patch needs to allow typical body movements without impacting the signal quality or the reliability of the patch’s adhesion to the skin. If the skin becomes irritated after a short period, this will typically disallow attaching another device to the irritated area, create patient discomfort and jeopardise patient compliance.
The smart adhesive patch concept for next-generation wearables relies on advanced materials that are being developed to provide improved electrode life, more data stability, better moisture control and increased patient comfort. Silicone elastomers, when extruded in films, offer tuneable wearability and excellent conformability, as well as stretchability, comfort and biocompatibility. Ergonomically, a smart patch should be so comfortable that the patient can forget they are wearing it in normal everyday life.
Silver, silver/silver chloride and carbon-based electrically conductive inks and thermoplastic polyurethane (TPU) films also are used for signal transfer in medical device smart patches, as well as for smart, stretchable sports and fitness clothing.
The Nighthawk™ smart adhesive concept patch developed by Holst™ TNO (Eindhoven, the Netherlands) in conjunction with Celanese Intexar™ Inks and Films, and DuPont™ (DE, US) Liveo™ adhesives showcases the ability to provide a breathable, cushioning skin interface; dielectric insulation to protect signal quality; long-term wear with secure, skin-friendly, silicone-based adhesion; comfort and conformability with stretchable materials; and secure connections for biosignal reliability.
The patch maintains a minimum distance between sensing electrodes for data stability and accuracy. Also, the liner system is designed to effectively attach the patch to the body without losing control of the pliable design. The 20% stretchability of the shape is unmatched by any other design on the market, as are other patient-centric attributes of the Nighthawk™ concept patch.
CONCLUSION
There are numerous parallels to be drawn from connected, wearable medical and diagnostic devices and the current evolution of connected drug delivery devices. The demands from the entire ecosystem are consistent – to reduce HCP interventions, deliver more personalised care for patients, maximise the potential of the data from such devices and ensure greater patient adherence.
Electronic inks are critical to the performance of wearable and diagnostic devices. As a proven, robust and compliant technology, they offer drug delivery manufacturers real opportunities for the development of even more innovative systemic therapies for diseases such as cancer and neurological conditions such as Parkinson’s, as well as chronic conditions such as diabetes.
The experience and expertise in wearable devices that resides in organisations such as Celanese can also enable drug delivery companies to develop more patient-friendly and comfortable wearable delivery systems that open the opportunity for further long-acting delivery applications.
REFERENCES
- “Remote Patient Monitoring (RPM) Market is Expected to Reach $175.2 billion”. MarketsandMarkets Research, Mar 3, 2023.
- “Wearable AI Market Size, Share & Trends Analysis Report By Type (Smartwatches, Smart Eyewear, Smart Earwear), By Application, By Operations, By Component, By Region, And Segment Forecasts, 2023 – 2030”. Grand View Research, accessed Jun 2023.

