To Issue 170
Citation: Watts A, Ahemed Y, Dujovny G, “Manufacture of Moisture-Sensitive Powders for Inhalation”. ONdrugDelivery, Issue 170 (Apr 2025), pp 52–56.
Alan Watts, Yusuf Ahmed and Gabriela Dujovny discuss manufacturing methods for inhalation powders and explain how moisture protection is an essential consideration throughout the manufacturing process.
Inhalation powders have been used for decades to treat lung diseases, as well as conditions requiring rapid systemic therapy. Patients benefit from dry powder formulations because they can often be dosed in a single breath, use devices that are pocket-sized and do not require refrigeration. Manufacture by spray drying has improved on traditional blended formulations by enabling higher-loaded doses and reducing oropharyngeal deposition. However, these powders present new challenges for manufacture, specifically for handling the intermediate powder, which is poorly flowing and often sensitive to moisture. Additionally, these powders require encapsulation in specialised capsules, such as Qualicaps’ (Roquette) Quali-V®-I Extra Dry Hypromellose capsules to ensure product dryness and stability. With proven experience in commercial production of spray-dried products for inhalation, Catalent has the infrastructure in place to manufacture, control and release these speciality products effectively.1
SPRAY DRYING INHALATION POWDERS
Spray drying is a widely used technique in pharmaceutical and food industries to produce dry powders from liquid feedstock. It is the preferred method for engineering inhalable powders, offering the benefits of high potency and a high respirable fraction for both small- and large-molecule formulations in a continuous and scalable process. During spray drying, water (and any other solvent present) is rapidly driven off causing solute precipitation and particle formation. Excipients commonly used in spray drying of inhalation powders are often sugars (e.g. trehalose, sucrose) and polyols (e.g. mannitol), which can stabilise the API in a glassy matrix and impart stability to large molecules by replacing the hydrogen bonds that were occupied by water.
It is extremely important that control over process and facility moisture is maintained to stabilise these powders. Often, total water content within these powders will be below 5% w/w to maintain their physical and chemical stability, allow for sufficient powder flow during filling operations and, most importantly, aerosolise effectively when inspiratory flow is encountered.
Formulating and Collecting
Formulation approaches to inhalation powder can enable protection from ambient moisture, at least for short durations. When spray drying, functional excipients, such as leucine, can be used to create a hydrophobic shell that preferentially accumulates on the outer surface of the forming particle, improving moisture protection of the dried powder while also reducing particle surface energy and cohesion. While shell formers provide some moisture protection, it remains necessary to further protect spray-dried powder from high relative humidity (RH) environments during powder collection from the spray drier, as well as in subsequent manufacturing unit operations.
During the spray drying operation, dried powders are collected into intermediate containers where they will begin to cool. It is important, however, to protect these powders when drying conditions result in moisture-saturated air within the process, as these cooling powders can serve as a surface for water condensation. This can be achieved by jacketing collection vessels to maintain higher collection temperatures or by sweeping collection vessels with dry nitrogen gas.
POWDER ENCAPSULATION
Intermediate bulk containers (IBCs) are often used to collect and store bulk spray-dried powder before moving to the encapsulation unit operation. All IBC sampling for intermediate release should be conducted under low, controlled RH conditions.
“BY CONTROLLING THE SUITE ENVIRONMENT, THE CATALENT BOSTON SITE CAN PROVIDE ULTRA-LOW RH FILLING WHILE ALLOWING OPERATORS TO INTERACT WITH THE EQUIPMENT WITHOUT THE RESTRICTION OF CONTAINMENT.”
Low RH Control Within Encapsulation Suite
Encapsulation requires that the powder be fed into equipment, where it will come into contact with multiple surfaces and be exposed to the environmental air for extended periods, often several hours for the filling operation. Encapsulators, such as the Harro Höfliger Modu-C series (Figure 1), are designed to handle poorly flowing powders and enable accurate filling at low masses using drum-filling technology.2 Operation of this equipment in a low and controlled RH can be accomplished by using containment around the equipment or by controlling the environment of the entire suite. By controlling the suite environment, the Catalent Boston (MA, US) site can provide ultra-low RH filling while allowing operators to interact with the equipment without the restriction of containment.

Figure 1: Commercial-scale encapsulation (Harro Höfliger Modu-C MS, left) and blistering (Pharmaworks TF2, right) within Catalent Boston.
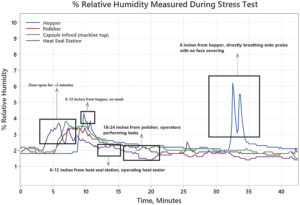
Figure 2: Relative humidity changes due to operator presence at various points in an ultra-low RH encapsulation suite.
To fully evaluate the environmental conditions that a powder might be exposed to during filling, a study was performed where a series of humidity probes were installed at key points throughout the encapsulation suite. Probes were placed at locations in the suite where operators might interact with the equipment or material: at the feed hopper inside the Modu-C unit; at the capsule polisher; on top of the unit at the capsule infeed; and at the bagged-capsule heat-seal station. While maintaining a 2% RH in the filling suite, these probes were monitored and challenged by various exposures that could potentially occur during normal operation. Operators were gowned as usual for normal GMP operation to test impact on local RH throughout the suite. As seen in Figure 2, the largest impact was a positive control where the operator removed their facemask, breathing 6″ away from one of the probes to cause a spike to 6% RH. When testing anticipated normal operations, opening the suite door for two minutes had the greatest impact, causing an increase in RH of all probes to between 3% and 4%. This testing demonstrated that operator presence in the suite might have some impact on RH; however, it is likely to be insignificant.
For an actual filling operation, probes remained in place for this three-hour process. With all operators present, baseline RH was maintained between 2% and 3% with minor spikes to ~4% RH, likely due to the door opening when operators entered and exited the suite. During the entire filling operation, RH was successfully maintained below 5%.
Low-Moisture Capsules
While controlled RH within the manufacturing environment is extremely important, capsule selection and handling can also greatly impact the protection of moisture-sensitive powders. Relatively high levels of moisture in traditional hydroxypropyl methylcellulose (HPMC) capsules (4–6%) or gelatin capsules (>10%), will transfer into a dry powder product during storage and should be avoided with moisture-sensitive formulations. Quali-V®-I Extra Dry Hypromellose capsules are specifically designed for inhalation applications, offering a low moisture content that is ideal for moisture-sensitive dry powder inhaler (DPI) formulations. These capsules, derived from standard HPMC Quali-V®-I inhalation-grade capsules, undergo an enhanced manufacturing process that reduces moisture to 2–3.5% w/w, while preserving their mechanical integrity for reliable drug delivery.
When Quali-V®-I Extra Dry capsules were tested under various mechanical stresses, the results showed that the reduced moisture content did not affect their structural integrity, performing similarly to normal HPMC capsules.3 Maintaining normal mechanical properties helps to ensure capsule integrity during encapsulation and blistering operations, as well as reliable aerosol performance during product release testing and patient use.
Like the powder intermediate, Extra Dry capsules can also adsorb moisture from the production environment. Thus, care should be taken to only expose these capsules to low RH conditions so that moisture is not adsorbed. Dynamic vapour sorption (DVS) run on 0% RH dried HPMC capsules shows a gradual uptake in moisture as the environmental RH is increased (Figure 3). Of note, at 40% RH (ambient RH), capsules equilibrate to a moisture content of >5% – outside of the specification limit for Extra Dry capsules – which has the potential to result in instability of a moisture-sensitive powder. It is critical, therefore, that Extra Dry capsules are handled, filled and packaged in a controlled environment that does not increase their moisture content.
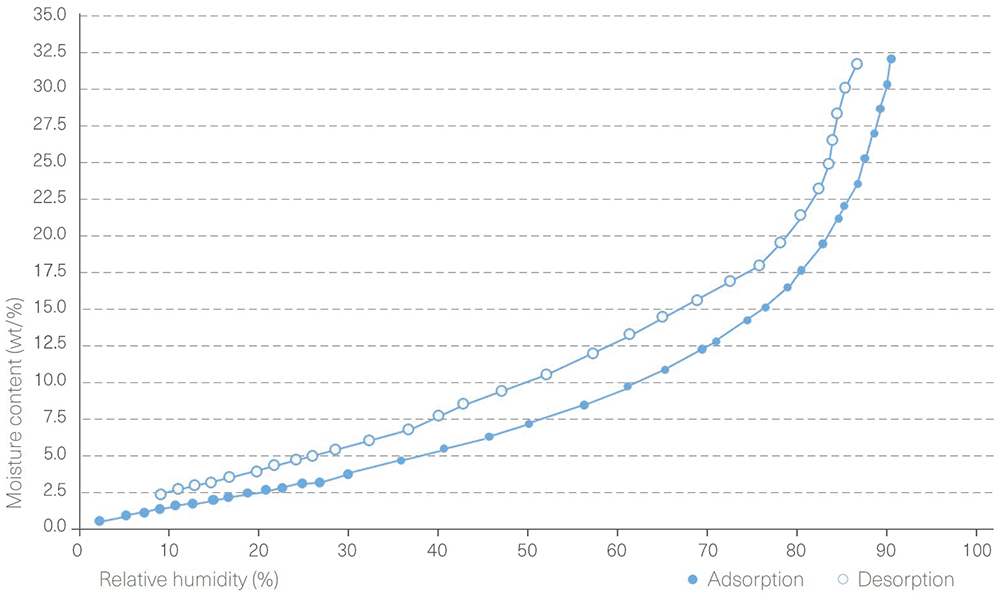
Figure 3: A DVS isotherm of an HPMC capsule.
A study was conducted to assess the effect of capsule moisture content on the mechanical properties of HPMC capsules used in DPIs. Capsules were conditioned at 11%, 34%, 54% and 0.5% RH, resulting in moisture contents ranging from 1.3% to 6.5% w/w. Puncture-resistance tests revealed that lower moisture content increased the puncture force slightly, with minimal impact on capsule puncture geometry (Figure 4).4 Even at very low RH (0.5%), capsules maintained their mechanical integrity, supporting their suitability for use in DPI products without fragmentation.
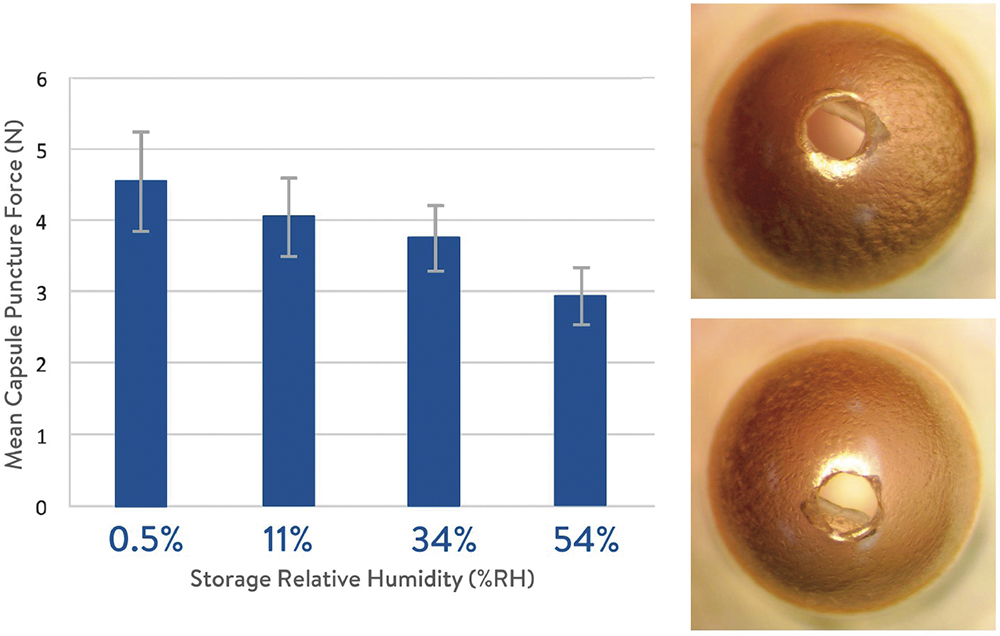
Figure 4: Puncture force change with various HPMC capsule storage conditions (left) and images of capsule punctures after 0.5% RH storage (right).
CAPSULE BLISTERING
Upon completion of powder encapsulation, moisture-sensitive products must then be blistered to allow for commercial packing and long-term stability. Like encapsulation, it is essential that this operation be performed under low RH so that filled capsules do not adsorb moisture from the manufacturing environment.
“CAREFUL CONSIDERATION OF THE INTEGRITY OF THE BLISTER SEAL IS NECESSARY, AS IT IS THE MOST LIKELY POTENTIAL PATHWAY FOR MOISTURE TO INGRESS.”
Blistering Materials
Materials and blister card design are critical to ensure protection from moisture. Cold-form aluminium cavities and aluminium lidding allow for superior moisture protection compared with polymeric cavities. Careful consideration of the integrity of the blister seal is necessary, as it is the most likely potential pathway for moisture ingress. Blister design elements, such as length from seal edge to cavity and seal integrity, can vary with heat and dwell time, and have been shown to have meaningful impact on the long-term stability of blistered moisture-sensitive material.5
In some instances, extra moisture protection is required and can be enabled by incorporation of desiccating materials within the blister card. Foils are available where a water-adsorbing polymer layer is embedded in the composite foil itself, while other solutions have used blister cavities intended to contain desiccant packs, which are connected via channels to drug product-containing cavities.
CONCLUSION
Moisture control is essential for nearly all tableted or powdered pharmaceutical products. For spray-dried inhalation products, protection from environmental moisture is essential throughout the manufacturing process and should be prioritised when selecting container closure materials, such as HPMC capsules or blistering materials. With several years of commercial manufacturing experience and proven facility controls in place, Catalent Boston has established a fit-for-purpose environment to spray, fill and blister moisture-sensitive powders.
REFERENCES
- Watts A, Tauber M, “ARCUS®: A Commercialised Technology Enabling High-Dose, Highly Respirable Powders”. ONdrugDelivery, Issue 158 (Apr 2024), pp 48–54.
- Berg C, Chin W, Goncalves P, “Smart Scaling for Successful Capsule-Based Dry Powder Inhaler Filling Using Drum Technologies”. ONdrugDelivery, Issue 154 (Nov 2023), pp 28–32.
- Coulman S et al, “Evaluating the Mechanical Properties of Extra Dry” Hard-Shell Capsules”. ONdrugDelivery, Issue 114 (Nov 2020), pp 12–16.
- Parker SA et al, “Determining the puncture performance of Quali-V®-I Hydroxypropylmethylcellulose capsules at very low moisture contents”. Proc, Respiratory Drug Delivery Europe 2019 (Estoril, Portugal), 2019.
- Stevenson CL, Bennett DB, “Development of the Exubera® insulin pulmonary delivery system”. Mucosal Delivery of Biopharmaceuticals, 2014, pp 461–481.

