To Issue 135
Citation: Lemasson O, Bourgeois S, Bourgeaux V, “”Nanoencapsulation: A New Era for Oral Solid Dosage Forms”. ONdrugDelivery, Issue 135 (Jul 2022), pp 16–20.
Oksana Lemasson, Sandrine Bourgeois and Vanessa Bourgeaux discuss the performance of the NanoMicS platform for the oral administration of nanoparticles.
“While widely used for injectables, nanoparticles remain poorly explored for oral administration.”
The oral route is the preferred route for drug administration because it allows better patient compliance. However, many factors limit the effectiveness of oral treatments, including the poor bioavailability of some APIs. It is estimated that 60% of new available APIs are rated as Class II and Class IV under the Biopharmaceutics Classification System (BCS) based on their low water solubility. Most of these APIs also have a low oral bioavailability due to extensive hepatic metabolism through cytochromes or high affinity with permeability glycoprotein (P-gp), a transmembrane transporter present in the intestine capable of pushing drugs back into the lumen, thus decreasing their absorption.1,2
Nanoencapsulation then appears to be a formulation of choice to overcome those biological barriers and achieve specific properties that are a key differentiating factor from other drugs. Nanoparticles act as a shield for the active molecule, preventing it binding to P-gp and cytochromes and conferring enhanced permeability.3 Nanoencapsulation can be applied to new chemical entities but also to already-on market APIs (through product lifecycle management). In this latter case, the nano approach gives pharmaceutical companies the opportunity to increase the rentability of active ingredients by developing “premium” drug product generation with enhanced therapeutic efficacy and minimised off-target side effects.
NANOMICS PLATFORM AT A GLANCE
To answer market needs, Skyepharma – which specialises in oral solid dosage forms and high-pressure homogenisation (HPH) technology – has taken the step to create a nanoformulation platform, NanoMicS, focused on the development of innovative nanoparticle-containing tablets, capsules or micropellets.
Skyepharma has already developed a first HPH-based process capable of producing nano-sized particles of APIs named IDD-Dissocubes™. This top-down technology, resulting in nanocrystals fof API, is currently on the market for the treatment of for lipidic disorders (Triglide®)4 and has allowed a dramatically reduced food effect and administered dosage of fenofibrate, compared with traditional oral dosage forms. Reinforcing the pipeline of bioavailability-improving solutions with nanoparticles manufactured through HPH isthus completely in line with Skyepharma’s strategy to bring high-value oral solutions to customers and ultimately patients.
While widely used for injectables, nanoparticles remain poorly explored for oral administration. Industrial challenges to be met include maintenance of nanoparticle features upon drying and tableting processes, process reproducibility during scale-up, implementation of relevant in-process controls, achievement of satisfactory holding time of nanomaterial and development of efficient cleaning procedures. A good understanding of the natural fate of nanobodies after release from solid forms, the identification of the pathways used to cross the gastrointestinal tract and the elucidation of the mechanism of release of the API from the nanoparticle are areas to be further investigated and mastered to ensure success of the “bench-to market” oral nanoplatform.
Therefore, the NanoMicS platform was developed in partnership with Claude Bernard University Lyon 1’s Laboratory of Automatic Control, Chemical and Pharmaceutical Engineering (LAGEPP) – which is highly experienced in nanoparticles and oral formulation processes. The platform started with the development of a generic nanoformulation to improve the solubility of lipophilic drugs, but there are plans to expand the portfolio of nanoformulations further to address all types of molecules, including temperature-sensitive APIs. As ecology matters, all formulations are developed with “green” processes, avoiding the use of volatile organic solvents detrimental to the environment.5
“The manufacturing of nanoproducts requires the control of nanomaterial properties such as size, shape, charge, composition, physicochemical properties and drug-release kinetics.”
RECENT SCIENTIFC ADVANCES
First results generated by researchers from LAGEPP on the nanoencapsulation of BCS Class II or IV APIs in solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) were very promising. This innovative strategy, improving the API’s solubility in digestive fluids and giving better control of its release and metabolism issues, also has the advantage of being biocompatible and biodegradable. Both SLNs and NLCs contain solid lipid, liquid lipid (Maisine® or Capryol® 90) and surfactant but differ in the whole structure, SLNs presenting a brick-wall structure while NLCs feature a hybrid structure (Figure 1).
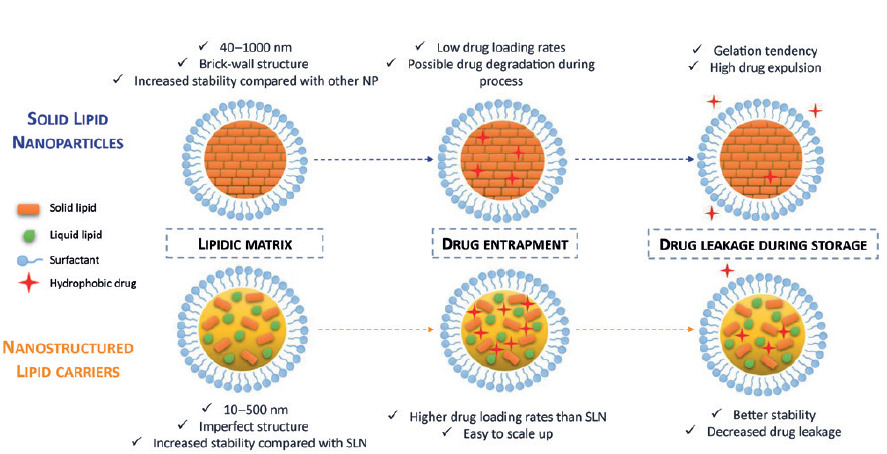
Figure 1: Main characteristics of SLN and NLC.
For the proof of concept, the selected model drug was spironolactone (SPI), a BCS class II API. The formulation of lipid nanoparticles was developed according to a previous study carried out at LAGEPP by Dumont et al,6 with a nanosuspension composed of two phases: the lipidic (including dissolved SPI) and the aqueous with surfactant and deionised water.6 The two phases were heated separately and homogenised by high shear agitation. This pre-emulsion was inserted in the Microfluidizer® LM20 (Microfluidics, MA, US) and pumped through the system, inside which high shear forces are applied to the emulsion.
The manufacturing of nanoproducts requires the control of nanomaterial properties such as size, shape, charge, composition, physicochemical properties and drug-release kinetics. Both SLNs’ and NLCs’ blank formulas provided satisfactory particle size characteristics, with a mean diameter below 200 nm and polydispersity index (PDI) below 0.2 (Table 1).
| Property | SLNs | NLC-C90 | NLC-MAI |
| D50 (nm) | 192.84 ± 13.92 | 168.22 ± 32.94 | 143.37 ± 0.49 |
| PDI | 0.174 ± 0.026 | 0.180 ± 0.042 | 0.184 ± 0.018 |
Table 1. Particle size characteristics of blank SLNs and NLCs (n=3), mean ± SEM.
Their observation with transmission electron microscopy (TEM) demonstrated they were all spherical shaped (Figure 2), which is a critical attribute of nanoparticles to cross the gastrointestinal tract. Compared with the blank nanoparticles, the SPI encapsulation did not lead to a significant difference in particle size. However, as shown in Table 2, the NLC formula with Maisine® (NLC-MAI) allowed the highest encapsulation, with 72.8% of SPI encapsulation efficiency, compared with 50.2% for NLC with Capryol® 90 (NLC-C90) and 60.7% for SLN.
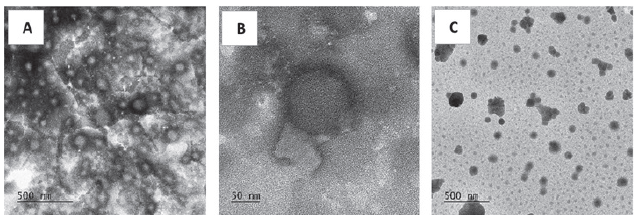
Figure 2. SLN (A&B) and NLC (C) observation by TEM.
| Properties | SLNs | NLC-C90 | NLC-MAI |
| D50 (nm) | 183.74 ± 33.39 | 184.97 ± 59.02 | 176.50 ± 37.53 |
| PDI | 0.167 ± 0.055 | 0.213 ± 0.070 | 0.217 ± 0.053 |
| EE (%) | 60.7 ± 5.3 | 50.2 ± 18.2 | 72.8 ± 1.7 |
Table 2. Particle sizes of SPI-loaded nanoparticles (n=9) and encapsulation efficiency (n=3) for the three types of lipid nanoparticles (SLN, NLC-C90 and NCL-MAI), mean ± SEM.
A stability study was conducted to ensure a sufficient holding time of the nanosuspension between its formulation and the drying. During seven days at room temperature, the general aspect remained unchanged. Concerning the particle size features, the mean diameter of the lipid nanoparticles increased slightly (from 171 nm to 216 nm, n=3) whereas the polydispersity index was stable (nonsignificant decrease from 0.18 to 0.16, n=3). These acceptable results confirm that the nanosuspension is stable under conventional storage conditions, without the need for specific precautions. Thus, the demonstrated stability of the nanosuspension allows few days between its formulation with the Microfluidizer® LM20 and its drying for compression.
“The proof-of-concept confirmed that HPH can be used to manufacture lipid-based nanoparticles with mean diameters lower than 200 nm.”
Drying the nanosuspension presents a double strategic interest. On the one hand, it is well described as an effective way to significantly improve the long-term stability of lipid nanoparticles. On the other hand, this process allows the obtention of powders, which will be compressed into tablets, to offer an innovative oral solid dosage form for patients.
Two drying techniques (spray-drying and wet granulation) were tested to produce an easily compressible and redispersible powder. For both techniques, the nanosuspension was mixed with suitable excipients until the obtention of a satisfactory general aspect of the powder. The two types of dried powder presented satisfactory flow properties, according to the European Pharmacopoeia (even “excellent” for the granulated batch) and were easily and successfully dispersed in water.
The powder obtained by wet granulation (Figure 3a) was selected for the preliminary compression study. Two batches of tablets were manufactured, at lab scale (total weight = 150 g) and pilot scale (total weight = 1000 g, Figure 3b). The tablets were easily dispersed in water, with features comparable to the initial powder. Moreover, all the units were compliant with the European Pharmacopoeia monographies relative to tablets (mass uniformity, disintegration time, hardness and friability), without any influence of the batch size.
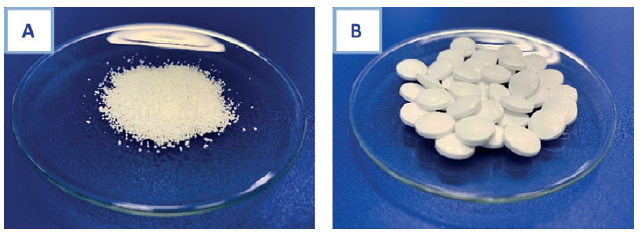
Figure 3. Granulated powder (A) used to produce lipid nanoparticle-based tablets (B).
In short, the proof-of-concept confirmed that HPH can be used to manufacture lipid-based nanoparticles with mean diameters lower than 200 nm. While encapsulating the model drug (spironolactone), the addition of a liquid lipid did not have a significant impact on the particle size but significantly improved the entrapment efficiency of the API, especially with the oil Maisine®. In vitro investigations are underway to study the release profile of the API as well as the improvement of its cellular permeability when encapsulated in lipid nanoparticles.
“Defining clearly the attributes of raw materials, their behaviour throughout the process and their quality impact on the final product is also a prerequisite to the successful development of nanoparticles.”
INDUSTRIAL SCALE-UP AND QUALITY-BY-DESIGN APPROACH
As a CDMO and centre of excellence in oral solid dosage form development and industrialisation, Skyepharma is highly experienced in quality-by-design (QbD) and process industrialisation, which are two strong assets for the development of the NanoMicSplatform. For nanoparticle-containing tablets, attributes such as nanoparticle size, API encapsulation efficiency, polydispersity index and drug-release kinetics come on top of critical quality attributes that are standardly evaluated for tablets (hardness, friability, mean mass and disintegration). To increase manufacturing robustness, the process analytical tool approach should be considered at the industrialisation phase to allow real-time product analysis and continuous feedback on manufacturing.
Defining clearly the attributes of raw materials, their behaviour throughout the process and their quality impact on the final product is also a prerequisite to the successful development of nanoparticles. In the case of solid lipid excipient, for example, changes in surface charge or morphology can alter the therapeutic properties of the API, and its characterisation is as important as the one of the API. Considering those industrial challenges early in development is a key element to reduce drug product development timelines and costs.
CONCLUSION
Skyepharma has made the strategic choice to establish itself as a pioneer in the administration of oral nanoparticles. Co-developed with LAGEPP, the NanoMicS platform aims to offer biocompatible delivery system solutions for BCS class II and IV molecules and reduce the number of leads of high therapeutic potential that are given up due to poor solubility issues. The Microfluidizer® technology appears as an eco-friendly solution to enhance oral bioavailability, broadening the spectrum of active molecules that could reach the market in oncology, immunology or infectiology and making patients’ daily lives easier.
REFERENCES
- Scioli Montoto S, Muraca G, Esperanza Ruiz M, “ Solid Lipid Nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects”. Front Mol Biosci, 2020, Vol 7, article 587997.
- Dumont C et al, “A proof-ofconcept for developing oral lipidized peptide nanostructured lipid carrier formulations”. J Drug Deliv Sci Technol, 2019, Vol 54, article 101394.
- Patil D et al, “Nanostructured lipid carriers: a novel targeted drug delivery system”. Int J Pharm Sci, 2020, Vol 11 (10), pp 4784–4793.
- Triglide website: http://triglide.com/, accessed Jul 2022.
- Palanivel G et al, “Microfluidization trends in the development of nanodelivery systems and applications in chronic disease treatments”. Int J Nanomed, 2018, Vol 13, pp 6109–6121.
- Dumont C et al,“In vitro evaluation of solid lipid nanoparticles: ability to encapsulate, release and ensure effective protection of peptides in the gastrointestinal tract”. Int J Pharm, 2019, Vol 565, pp 409–418.

