Citation: Mayer T, “Next-Generation Wearable Delivery Device for Large-Volume Biologics”. ONdrugDelivery Magazine, Issue 105 (Feb 2020), pp 56-59.
Thomas Mayer discusses Sonceboz’s platform of wearable injection devices for large-volume biologics, which can follow drugs from clinical trials to commercial phase through lifecycle management, which reduces risk and speeds time to market.
Demand for biologics such as monoclonal antibodies continues to bring growth opportunities to the large-volume wearable injectors market, with some researchers estimating the global market to reach US$1.5 billion (£1.2 billion) by 2030. Interest in the devices is being driven by their ability to deliver high volumes between 3 mL and 20 mL – or higher – into subcutaneous tissue. The sweet spot regarding delivery volumes seems to be around 10 mL.
“The wider the span of applications, the more challenging it is to find the right technical solution.”
Novel drug delivery technologies such as co-formulated hyaluronidase go some way to making high volume delivery possible. But delivery of high volumes of drugs into subcutaneous tissue – especially when intended for home use/self-administration also requires new delivery device technologies, such as on-body injectors, so as not to limit a patient’s freedom to live life.
MULTIPLE DRUGS, ONE TECHNOLOGY SPEEDS TIME TO MARKET
From a platform point of view, in an ideal world, a pharma company with multiple drugs would want one technology to leverage across different drug products. But the wider the span of applications, the more challenging it is to find the right technical solution.
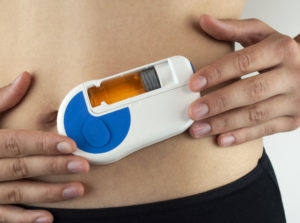
Figure 1: Sonceboz’s electromechanical wearable injector is designed around an omnidirectional pump module.
Sonceboz is currently developing an electromechanical wearable injection device platform (Figure 1) designed around an omnidirectional pump module. This makes it possible to connect to various primary drug containers of capacities up to 20 mL or even higher – such as vials or cartridges – without the need for changing the overall system architecture. This is possible by separating the pump module from the drug reservoir – making the two independent of each other.
The advantage of this is that one can rely on one proven system architecture but also leverage already established and proven primary drug containers – and thereby accelerate development and time to market.
These characteristics are important in achieving the goal of having a drug delivery platform that a pharma company can use from clinical trials to commercial scale to lifecycle management, and have confidence that the system is reliable, taking a level of risk out of development.
By offering different delivery volumes, programmable pump delivery profiles and container options (Figure 2), pharma companies can multiply the platform across different drug products. The advantage is reliance on one proven system, thereby accelerating development and time to market.
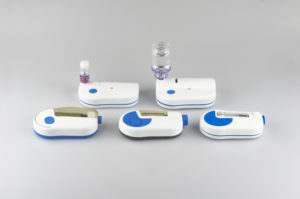
Figure 2: The Sonceboz wearable injector platform offers different delivery volumes, programmable pump delivery profiles and container options.
THE FAMILY OF WEARABLE INJECTION DEVICES
Sonceboz devices are designed to be easy for patients to use, and device prep is completed by a series of simple and intuitive steps. All the wearable injection devices use the company’s electromechanical pump system with dynamic soft-cannula insertion. The Sonceboz platform includes five single-use dedicated variants that enable use from clinical trials to lifecycle management.
The large-volume injector (LVI) comes in two versions. One is cartridge-based, whereby the user inserts a prefilled cartridge into the device at the point of use. This version comes in different sizes to accommodate cartridges from 3 mL to 20 mL. The second LVI variant has an internal reservoir and uses a standard vial adapter that automatically transfers the contents of the vial or syringe inside the on-body device prior to injection. A temporary drug container inside the device is designed for short-term storage. This device is intended for clinical trials or cases where a pharma company wants to keep the vial as a primary container but still provide a delivery device solution to its patients.
“The dual-cartridge injector (DCI) uses the same pump architecture
to independently inject APIs from two separate containers, either sequentially or simultaneously.”
The LVI-V is for vial transfer with standard vials/syringes up to 20 mL; the LVI-U is for prefilled cartridges up to 20 mL and is optimal when cold storage is required since it allows for separate storage of drug and device, which in turn reduces the package footprint; and the LVI-P is a prefilled, preloaded version.
The dual-cartridge injector (DCI) uses the same pump architecture to independently inject APIs from two separate containers, either sequentially or simultaneously. This is an ideal solution for combination therapies or lifecycle management where the API is already filled in a smaller volume cartridge such as a standard 3 mL. DCI is well suited for combination therapies in oncology or in cases when one wants to maintain an existing drug container but double the delivered payload.
The auto-reconstitution injector (ARI) is intended for automatic reconstitution of lyophilised drug products. In biologics, there is often a need to store a drug in a powdered state because it will not remain stable over its intended shelf life in a solution. But manual reconstitution of lyophilised drug formulations is a complex procedure and challenging for caregivers and even more so for patients.
With ARI, a prefilled and loaded diluent is automatically transferred into the vial containing the lyophilised drug product. When the transfer is complete and the drug reconstituted, the pump retransfers the resolved drug product back into the device. Once this step is completed, the vial adapter is removed and the device used on-body. This simplifies the use of lyophilised products and supports self-administration. A built-in sensor suite monitors proper handling and orientation during transfer and mixing.
The entire Sonceboz wearable platform comes with built-in connectivity using Bluetooth Low Energy as the communications and connectivity interface. This enables pharma companies to include data from the on-body devices into a dedicated digital-health solutions suite.
INCORPORATING THE MOTION OF MECHATRONICS
Mechatronics is what makes the Sonceboz devices so flexible. Mechatronics is at the crossroads of electronics, mechanics and computing, with a variety of applications. For more than 25 years, millions of mechatronic drives from Sonceboz have been used in hospitals, clinics and laboratories around the world in the form of blood pumps and syringe pumps for dialysis machines, and motors or actuators for diagnostic equipment. The company produces 70 million mechatronics systems annually, all of which are manufactured in Switzerland.
Now, the company is applying precision mechatronics to its wearable injection device platform in the form of its GentleTouch piston pump. Its integrated three ports allow drug transfer and mixing across a range of delivery volumes, speeds and viscosities without the need for design changes.
The electronically driven pump goes through two stages during its cycle. In the first stage, the pump fills itself by applying a vacuum to whatever primary container it is connected to. The second phase uses positive displacement to pump the drug into the patient or, if required, to another container. The fluid path is completed by the dynamic needle insertion and retraction system, which features a thin 27G soft cannula and built-in needle-stick injury prevention.
This design and the ability to command the direction of flow allows for the previously described flexibility in terms of use cases and therapeutic applications. The pump system is independent of the drug container so one can connect both small and very large containers. And the pump is constructed with materials and coatings that are compatible with large-molecule biologics; the pump’s lubrication system has been designed to be in line with biologic drug product requirements. Also, the motion that the drug fluid is subjected to is not damaging to the drug product.
LEVERAGING AUTOMOTIVE EXPERIENCE IN PHARMA AND MEDTECH
Situated in the heart of the Swiss Watch Valley, since 1936, Sonceboz has been focused on mechatronics, beginning with industrial time-keeping equipment and transforming into a leading supplier of actuation for the passenger and commercial vehicles industry.
Adherence to stringent quality standards and cost pressures in the auto industry is something Sonceboz has been able to leverage in the medical device sector, and now in pharma drug delivery. In the auto industry, it is expected that a mechatronic system function 100% of the time, and Sonceboz leverages its expertise in high-quality and high-volume automated manufacturing to provide the utmost safety and reliability standards to drug delivery devices.
The wearable injector platform follows design for manufacturing (DFM) guidelines, enabling top-down automated assembly of all components at top quality. And, by implementing complete end-of-line control, every functional element of the wearable platform is then tested according to specifications to ensure the device performs its job as intended from a technical point of view. From a user point of view, the device is intuitively designed so that a user will know how to use the unit just by looking at it.
Sonceboz is currently in the engineering verification phase with the platform to fully characterise the design prior to design verification testing. The device platform is expected to be ready for clinical trials in late 2021. The future of drug delivery is technology that allows patients to receive their medication at home, not in a hospital or outpatient clinic. Mechatronics, building on a long and rich history, is writing a new chapter in the medical field, particularly in drug delivery.

