To Issue 170
Citation: Conte S, Regard A, “Nose-to-Brain Delivery: Patient-Centric Device Development and Performance Testing”. ONdrugDelivery, Issue 170 (Apr 2025), pp 36–39.
Sophie Conte and Alain Regard describe Nemera’s journey in nose-to-brain device development, highlighting the evaluation tools, methods and usability testing developed in-house to steer innovative device design and engineering.
The nose has been used as a portal to deliver therapeutic substances for thousands of years. In Amazonian cultures, simple handmade tools are used to administer pharmacologically active “rapeh” powders to the nose for ceremonial and medicinal purposes. In contemporary medicine, the intranasal devices used to deliver synthetic therapeutic compounds are technologically intricate. Nonetheless, achieving consistent and effective nose-to-brain (N2B) drug delivery remains a complex challenge.
MULTI-DIMENSIONAL CHALLENGES IN N2B DRUG DELIVERY
Nasal drug delivery is a promising route for direct brain targeting, especially in central nervous system (CNS) disorders and neurodegenerative diseases, such as Parkinson’s and Alzheimer’s diseases, as well as some brain cancers.1 Drug development pipelines include small molecules, proteins, peptides and nucleic acid-based candidates. Today, achieving clinically effective N2B drug delivery is close to becoming a reality thanks to the multidisciplinary approach integrating pharmaceutics, pharmacology and ergonomic device engineering to overcome numerous challenges.
Formulation Challenges
Formulation-based drug delivery technologies, such as functional excipients, complex formulation and processing technologies, feature in the multifaceted approach in systemic and direct-to-brain nasal delivery. Formulations are designed to protect the API (e.g. encapsulation); increase residence time (e.g. muco-adhesive and thermogelling excipients); and enhance permeation, absorption and uptake into transport systems and tissues (e.g. solubilisers and permeability enhancers).
Formulation viscosity has a significant impact on deliverability to a precise target in the nasal cavity and on device performance. Ideally, an N2B delivery device must achieve consistent performance across a range of viscosities.
Anatomical and Physiological Challenges CNS and direct-to-brain drug delivery via the nose has gained significant interest and traction due to the identification and greater understanding of three different transport pathways:
- The Olfactory Mucosal Pathway: The olfactory cleft epithelium is unciliated and associated with reduced drug clearance and increased residence time. Absorption occurs through paracellular junctions leading to uptake into cerebrospinal fluid and transport to the brain, bypassing the blood–brain barrier (BBB). This pathway is purported to result in faster onset of clinical effects.
- The Trigeminal Nerve Pathway: The epithelium in this area is a major drug deposition target site for systemic drug delivery. It is highly innervated with trigeminal nerves, and may also be a minor pathway for CNS and brain delivery for drug compounds unable to pass the BBB.
- The Olfactory Nerve Pathway: The olfactory nerves in the upper nasal cavity provide a localised target for drug delivery; as these nerves transmit sensory information related to smell from the nasal cavity to the brain, it is proposed that this may constitute another gateway to CNS uptake and brain delivery.
For direct N2B delivery, the olfactory mucosal pathway is considered the most promising. However, achieving precise drug deposition in this small, inaccessible area – high-up, towards the back of the nasal cavity and approximately 7 cm from the nostrils – requires the design of a device with specific performance features with respect to plume characteristics, velocity and trajectory.
Usability Challenges in Intranasal Device Design
Usability and human factors engineering (HFE) studies are crucial for developing user-friendly and safe devices that foster patient adherence and acceptability. Devices that are uncomfortable, difficult to use or intimidating are less likely to be used correctly and consistently. These studies engage patients, caregivers and healthcare professionals to identify key design factors, such as comfort (minimise discomfort during insertion and dose expulsion), ease of use (intuitive design that simplifies administration and addresses dexterity issues) and safety of use (clear, concise instructions and training materials to ensure proper use).
“CREATING A DEVICE FOR N2B DELIVERY REQUIRES A MAJOR SHIFT IN DESIGN SPACE AND ENGINEERING.”
NEMERA’S DEVICE DEVELOPMENT APPROACH: FROM SPRAY TO GUIDED STREAM TECHNOLOGY
Creating a device for N2B delivery requires a major shift in design space and engineering. Nemera’s approach aims to develop an easy-to-use device capable of targeted, precise and acceptable drug deposition in the olfactory cleft, where:
- Targeted means delivering a volume of drug formulation with stable jet characteristics in an accurate trajectory
- Precise means ensuring that the trajectory of the formulation is reproducible, within an optimum range, by limiting variability in the device’s angle of insertion in the patient’s nostril
- Acceptable means minimising discomfort during insertion and dose expulsion, limiting backflow and irritation
- Easy-to-use means intuitive use during administration, minimising human error.
Nemera’s initial investigations have included a successful collaboration with Professor Ben Forbes of King’s College London (UK) in a clinical study that explored the intranasal delivery of insulin to the brain. Different configurations of Nemera’s standard nasal sprays were used to administer a liquid insulin formulation in healthy volunteers. The pharmacodynamic effects were detected by MRI scanning, and results indicated that a degree of insulin delivery to the brain was achieved with a standard nasal spray configuration. An in vitro nasal cast model with high-performance liquid chromatography analysis revealed quantitative and qualitative insights about intranasal insulin spray performance. While all spray configurations achieved high overall insulin deposition in the nasal cavity, the amount of drug reaching the olfactory cleft region was low.2
Results of this study and others led Nemera to design a specific device configuration with the aim of achieving >50% drug deposition in the olfactory cleft. Initial prototype devices have been refined, culminating in the “Guided Stream technology” for direct N2B delivery. Tests with the company’s latest prototype indicate a reproducible delivery trajectory and significantly higher targeted placebo formulation deposition in the target olfactory area.
Innovative Evaluation Approaches in Device Development
Evaluating prototype delivery devices in an emerging field, such as N2B drug delivery, is technically and intellectually challenging. There are relatively few novel device evaluation studies published in the scientific literature and no standard testing set-ups and protocols. This confounds comparative and correlative observation but also drives pragmatic solution finding and invention in design of experiment (DoE) approaches.
3D Nasal Cast Models
Research indicates that the conditions of intranasal device administration have the biggest impact on targeted drug deposition in the olfactory cleft. Therefore, an anatomically relevant 3D nasal cast is essential to evaluate the sensitivity of the device with respect to the variables of administration, including insertion depth and orientation.
Nemera has extensive knowledge in developing, validating and using different nasal casts in its R&D programmes, which has led to the development of a nasal cast specially adapted for N2B delivery and deposition studies, based on the work of Dr Jan Brüning of the Charité – Universitätsmediz in Berlin (Germany) and partners. Their approach to address the problem of inter-patient anatomical variability in research was to propose a standardised geometry representative of a healthy nasal cavity, generated using CT scans and segmentation data from 25 symptom-free subjects, coupled with statistical shape model techniques.3 These data were used to create a nasal cast model, 3D printed in a translucent, high-resolution polymer, to enable both qualitative (through visual observation and photography) and quantitative (through extraction and quantification of deposition) assessments.
“NEMERA HAS SHOWN THAT THE IMPACTION FORCE GENERATED BY GUIDED STREAM TECHNOLOGY IS SIMILAR TO THAT OF A COMMERCIAL PRESSURISED NASAL SALINE PRODUCT.”
In Vitro Investigation of Intranasal Device Performance and Drug Deposition
The nasal cast is the central tool for evaluating and quantifying drug deposition and for sensitivity studies that investigate the impact of device orientation, insertion depth and viscosity of the formulation, as well as “jet” characteristics on formulation deposition. This requires integrating the nasal cast with additional equipment in specialised experimental set-ups and protocols.
- Jet characterisation is associated with targeted drug delivery and is evaluated using Nemera’s optic bench set-up, which uses high-speed imaging equipment to assess jet width, jet cone, front speed and deposition efficacy.4 Custom software analyses video and image data of the actuation event and jet shot.
- Jet impaction force is an extremely important variable to measure, as it affects patient comfort and acceptability and must be optimised in device design. If too strong, the force of the jet hitting the nasal wall epithelium and olfactory area can cause discomfort. With limited research in this area, Nemera’s experimental set-up to measure jet impaction force is based on research by Guo et al (2009),5 which integrated an automated actuator in the nasal cast. Nemera has shown that the impaction force generated by Guided Stream technology is similar to that of a commercial pressurised nasal saline product, as well as to the data generated by Guo et al on nasal sprays.6

Figure 1. In vitro administration bench, double inclination base and automated actuation apparatus for intranasal device sensitivity studies.
- Deposition studies are performed with the nasal cast integrated with equipment for automated actuation, and a system for simulating physiological breathing can be added. The platform enables precise control of device orientation during administration to investigate the sensitivity of device orientation, insertion depth and viscosity of the formulation.
Nemera’s in vitro DoE for evaluating Guided Stream technology prototypes use the nasal cast set-up with a double inclination base and an automated actuator to investigate the range in which the combined administration parameters achieve the targeted drug deposition levels of >50% (Figures 1 and 2).
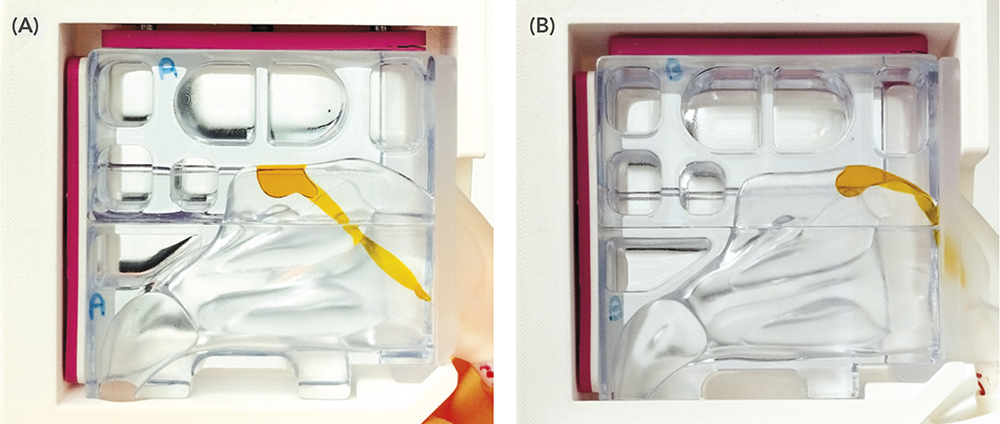
Figure 2. Performance of a prototype Guided Stream technology device delivering a placebo dyed formulation to the upper back (A) and upper front (B) of the target olfactory area in Nemera’s nasal cast.
Intranasal Device Usability and Human Factors Programme
Usability studies are designed to simulate real life use, and Nemera’s engineers have developed a wearable headset with orientation sensors on both the headset and the device itself. This equipment enables the monitoring of the device orientation relative to the patient’s head during simulated administration to explore variables that impact device administration and, therefore, clinical efficacy.
Voice of the Patient and HFE studies undertaken by Nemera Insight, (IL, US) were integrated in the early phases of device design. These studies revealed key user preferences about device portability, acceptable activation steps and features that maximise user confidence and comfort while minimising irritation. Nemera’s Guided Stream technology devices will be further evaluated in an HFE test programme later in 2025.
ON TARGET FOR N2B DELIVERY
To conclude, Nemera’s innovative and robust experimental approach has resulted in the development of Guided Stream technology devices and strengthened the company’s expertise in the field of device performance characterisation. Outcomes are extremely promising with prototype devices achieving consistently high levels of deposition in the target olfactory cleft area, with limited sensitivity to administration variables. Nemera’s devices are available for early phase clinical evaluation, and the company is confident that Guided Stream technology will, ultimately, contribute to the development of safe and effective treatments for patients.
REFERENCES
- Sedo KR, “Short Report 2024, Brain Targeting: The Final Frontier”. PharmaCircle, 2024.
- Wingrove J et al, “Characterisation of nasal devices for delivery of insulin to the brain and evaluation in humans using functional magnetic resonance imaging”. J Control Release, 2019, Vol 302, pp 140–147.
- Brüning J et al, “Characterization of the Airflow within an Average Geometry of the Healthy Human Nasal Cavity”. Sci Rep, 2020, Vol 10 (1), article 3755.
- Regard A et al, “Targeted drug delivery to the olfactory cleft: In-vitro deposition study using prototype jet nozzles”. Abstracts from The Aerosol Society, 34th Drug Delivery to the Lungs: (Edinburgh, UK) Dec 2023.
- Guo C et al, “Evaluation of impaction force of nasal sprays and metered-dose inhalers using the Texture Analyser”. J Pharm Sci, 2009, Vol 98(8), pp 2799–2806.
- Regard A, Berard L, “Evaluation of Impaction Force of a Jet for Targeted Nasal Drug Delivery”. Poster Presentation, 35th Drug Delivery To The Lungs (Edinburgh, UK), Dec 2024.

