To Issue 165
Citation: Merhige J, Thayer D, “Opportunity Through Innovation – Environmental and Social Sustainability in Injection Devices”. ONdrugDelivery, Issue 165 (Sep/Oct 2024), pp 18–22.
John Merhige and Dan Thayer discuss how the successful implementation of ESG principles in the pharmaceutical sector requires innovation that allows sustainability to be seen as an opportunity rather than a burden. Furthermore, the authors present the case that improving on the social aspect of ESG is just as important as improving on the environmental side.
Across all industries, forward-looking companies are aiming to implement more sustainable practices and create more sustainable products. In the face of the global climate crisis, and the major policy efforts of governments worldwide to tackle it, the move is becoming a necessity. However, it need not be a compromise – indeed, if more sustainable ways of doing and making things are going to take root, they should be seen as an opportunity to be seized, rather than an obligation to be met, with the potential for companies to reduce costs, environmental impact and societal burdens.
In some industries, the option exists to take a “move fast and adjust” approach to innovation. However, that is not the case in pharma. As a heavily regulated industry with a direct impact on the health of the patients it serves, the pharma industry is naturally conservative and risk-averse when it comes to radical or rapid change. However, this need for a steady, cautious approach does not exempt pharma from the need to innovate – the need for sustainability is here, and both its demands and its opportunities are immediate.
“Much like a proverbial oil tanker, the giants of the pharma industry turn slowly, so it is the role of smaller, emerging companies to present innovative technologies and approaches that can help their larger, more established partners adapt more swiftly to the needs of the moment.”
Much like a proverbial oil tanker, the giants of the pharma industry turn slowly, so it is the role of smaller, emerging companies to present innovative technologies and approaches that can help their larger, more established partners adapt more swiftly to the needs of the moment. Credence MedSystems understands this dynamic and is leading the way in implementing and facilitating environmental, social and governance (ESG) practices with its “Innovation Without Change” approach. Credence’s technology is designed to work seamlessly with current pharma practices and infrastructure, making it easy to adopt and incorporate into existing production processes, while also providing the benefits of increased environmental and social sustainability.
ACHIEVING ENVIRONMENTAL SUSTAINABILITY IN PHARMA
When most people discuss sustainability, it is the environmental aspect of ESG that first comes to mind. This is a critical area for the pharma industry to improve on, but doing so presents a number of significant challenges; any changes made within pharma must first be looked at through the lens of patient safety, which must always be the top priority. Because of this, certain obvious approaches to improving environmental sustainability, such as recycling, are uniquely difficult for pharma.
While some pilot schemes have been trialled for recycling non-invasive delivery devices, such as inhalers, this is significantly more problematic for injectable therapies, as needle reuse and needlestick injuries pose a significant hazard to the health of patients, caregivers and healthcare professionals – as well as the wider population if the devices are not properly disposed. Therefore, any injection system, even if there is a reusable element to it, is going to include a disposable element by necessity.
“This anticipated market growth means that minimising the environmental impact of injection devices is a pressing concern for the industry.”
As such, injection system developers must consider alternative approaches to reducing the environmental impact of their products. Compounding this, injection device usage is expected to increase significantly due to the massive growth of GLP-1 analogues for the treatment of obesity and diabetes – with GLP-1 users in the US forecast to reach 30 million by 2030.1 Overall, the global self-injection device market was valued at US$6.5 billion (£4.9 billion) in 2023, with an anticipated compound annual growth rate (CAGR) of 5.11%,2 while the global prefilled syringe market is expected to achieve revenues of $7.1 billion in 2024, growing to $13.1 billion in 2030 at a CAGR of 10.8%.3 This anticipated market growth means that minimising the environmental impact of injection devices is a pressing concern for the industry.
There is, however, a natural solution to this conundrum – minimising the material and size used in and by these injection devices. This approach makes sense not only from a sustainability perspective but also from a business standpoint. Reducing the material used in a drug delivery device will reduce its overall mass. This, along with innovative design, can minimise the volume occupied by the device. Taking these steps can have follow-on benefits throughout the product lifecycle.
For example, reducing mass and volume has a huge impact on logistics, where lighter products reduce the fuel consumption required to transport them and smaller products mean that more can be transported in the same vehicle, which is especially valuable for products requiring cold chain storage – approximately 35% of the market in 2022.4 Another major consideration here is secondary packaging; smaller products require less packaging, which further reduces the material requirements of the overall product. The global pharmaceutical secondary packaging market was valued at $6.2 billion in 2022.5 It is easy to see how these factors cascade and compound on each other, combining to produce meaningful reductions in environmental impact and, crucially, total cost of ownership for the end product.
In this way, reducing the material requirements of a device is an unequivocal good for pharma companies. Unlike some other approaches, such as switching to equivalent amounts of more environmentally friendly but more expensive materials, or purchasing credits from carbon or plastic offsetting schemes,6 material reduction is not a compromise between sustainability and profitability; rather, it is a boon to both at once, which is vital to ensuring that sustainability programmes succeed and become embedded within companies’ cultures and practices.
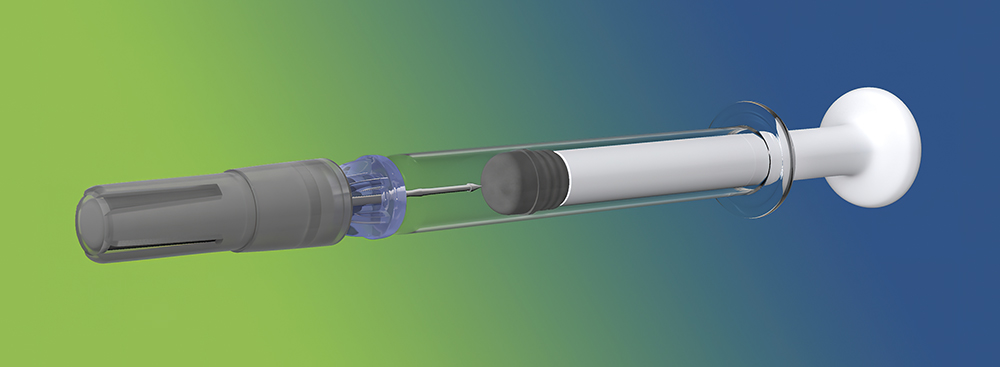
Figure 1: The Credence Companion® Safety Syringe System.
INNOVATION WITHOUT CHANGE IS THE WAY FORWARD

Figure 2: The Credence Companion syringe with the needle assembly mounted on a standard syringe barrel to provide integrated needle safety.
The Credence Companion® Safety Syringe System (Figure 1) is a perfect example of this in action. To combat the pervasive hazard of needlestick injuries in healthcare, many device companies have developed needle safety systems that automatically secure the needle after use. Typically, these systems involve a bulky add-on assembly around the syringe that significantly increases material use, volume and weight. The Credence Companion, on the other hand, employs an integrated approach that retracts the needle into the plunger rod at the completion of the injection, achieving the benefits of a passive needle safety system without significantly adding to the material, weight or footprint of a standard syringe barrel (Figure 2).
“The Companion was shown to eliminate 54% of the weight of added components, use only 40% of the plastic, occupy 47% of the volume pre-use and occupy 33% of the volume post-use.”
In a study comparing the Companion 1 mL long format to a leading conventional add-on safety device, the Companion was shown to eliminate 54% of the weight of added components, use only 40% of the plastic, occupy 47% of the volume pre-use and occupy33% of the volume post-use (Figure 3). Reducing the post-use footprint by two thirds carries the added benefit of a smaller volume needing to be disposed of, making more efficient use of the space in sharps containers and therefore reducing the number of sharps containers used and labour associated with changing them. Furthermore, compatibility with industry standard syringe barrels and standard nest-and-tub formats enables pharma companies to use the barrels they prefer and maintain their existing filling processes.
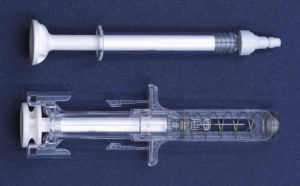
Figure 3: A typical add-on needle safety system (bottom) compared with the Credence Companion with its integrated needle safety (top).
Another clear example where compromise is not required to achieve sustainability is the Credence Dual Chamber Syringe System, which is used to deliver drugs that require constituents to be kept separated during storage. The most common approach to addressing this challenge is to use a vial kit that includes numerous vials, syringes, needles and other components in a large tray and package (Figure 4). This approach presents obvious sustainability challenges, as well as usability challenges that risk the successful and safe dosing of the medication.
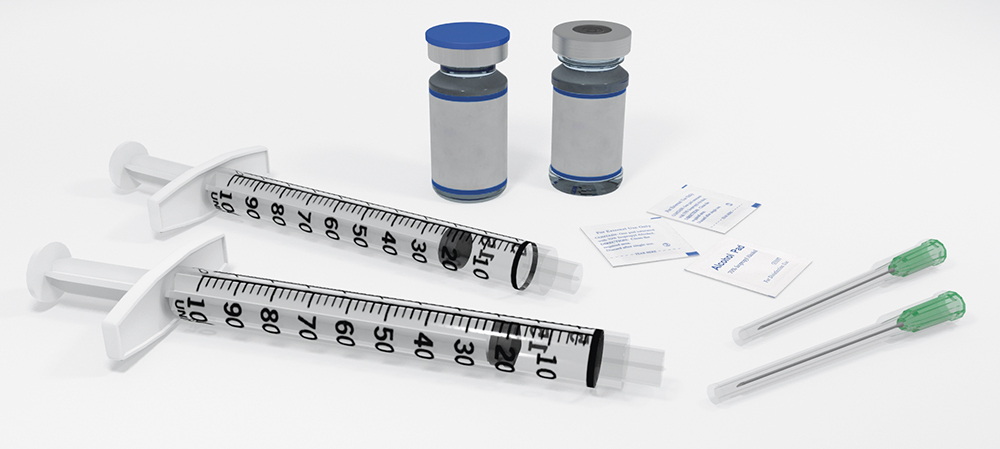
Figure 4: A standard multi-vial reconstitution kit.
Older dual-chamber technologies are also an option, although they are traditionally large, costly and complex devices that are difficult to manufacture and even more complex to use. The Credence Dual Chamber system is different. At first glance, the Credence Dual Chamber system looks like an ordinary syringe; it uses a standard syringe barrel with Credence’s internal technology dictating the flow of liquid. The Credence Dual Chamber system can enable either:
- Reconstitution of a liquid from the rear chamber into another liquid or solid in the front chamber prior to injection, or
- The sequential delivery of two liquids.
In each option, the system allows a familiar, user-friendly and ready-to-inject format (Figure 5). Furthermore, the Credence Dual Chamber system includes the same integrated passive needle safety features of the Credence Companion.
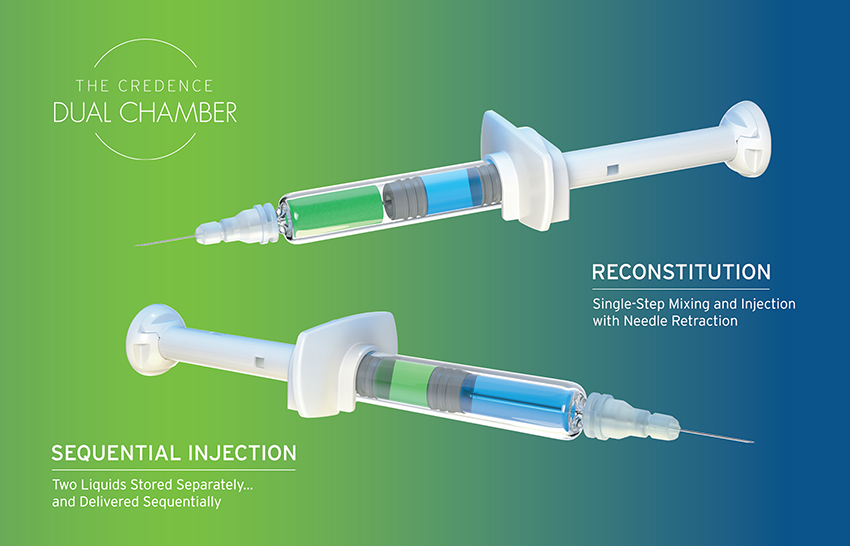
Figure 5: The Credence Dual Chamber Syringe System enables either reconstitution or sequential injection.
Both these systems are emblematic of Credence’s Innovation Without Change approach. By leveraging current prefilled syringe manufacturing infrastructure and assimilating into existing filling lines, the Credence Companion technology does not necessitate a significant shift in pharma manufacturers’ current processes, keeping the cost of adoption low and obviating the environmental impact of building and installing extensive machinery for new production lines. Ensuring compatibility with current syringe barrels and manufacturing infrastructure/processes reduces the cost of ownership, minimises the risk of adoption and smooths the path to market.
In fact, Credence’s Innovation Without Change philosophy extends beyond its technology and into its production and logistics strategies as well. The company understands that continuity of supply is crucial in pharma, and so it partners with well-established and trusted contract manufacturing organisations for the commercial manufacturing of its Companion and Dual Chamber systems. This ensures continuity of supply and integrates seamlessly into the existing business models of the pharma industry, enabling partners to take advantage of Credence’s innovations without changing their own operating procedures.
Holistically, Credence’s technology and strategic approach enable both sustainability improvements and cost reductions across the value chain. By offering injection systems that fit within the footprint of existing prefilled syringes, not only does Credence directly reduce the material requirements and cost of ownership of these systems, it reduces the logistical burden as well. By applying Innovation Without Change thinking to environmental sustainability and taking a material reduction approach, sustainability becomes an opportunity rather than an obligation.
“By aiming to better serve the needs of these stakeholders and reducing the burdens that treatment places on them, the industry can produce better, more desirable products and create opportunities for numerous advantages.”
MORE THAN JUST THE ENVIRONMENT – SOCIAL SUSTAINABILITY
Often, environmental considerations are both the beginning and end of a discussion about sustainability. However, while carbon footprint and material usage are critical aspects of sustainability, it would be a major mistake to neglect the social aspect of ESG. Ultimately, patients and healthcare professionals are at the core of the pharma industry – by aiming to better serve the needs of these stakeholders and reducing the burdens that treatment places on them, the industry can produce better, more desirable products and create opportunities for numerous advantages, such as increased patient adherence, reduced cost of healthcare and improved outcomes.
One of the biggest challenges currently facing the drug delivery and pharma industries is that of low patient adherence to their therapies. Low adherence results in a worsening of patients’ conditions and increased hospital admissions, putting a greater strain on global healthcare systems. Taking a social sustainability approach is key to tackling problems such as adherence – by reducing the burden of treatment on patients, it makes it easier for them to remain adherent, and therefore more likely that they will do so.
There are already trends within the industry leading in this direction. One of the most important of these is the trend towards at-home self-administration of injectables, which gives patients greater autonomy, reduces the need for travel to clinics and lessens the burden on healthcare professionals. However, self-injection presents its own challenges for patients – they are frequently non-expert users and must be responsible for proper and safe storage, complex preparation (sometimes including mixing and reconstitution), accurate dosing and the safe disposal of potentially dangerous sharps waste.
Credence’s technology provides ready solutions to these challenges. At the heart of Credence’s design philosophy is the fact that patients are people, so delivery devices must be designed with people in mind. By integrating Credence technology into a standard syringe with a novel approach where the mechanism of action provides important user cues, the Credence Companion and Dual Chamber systems achieve multiple social sustainability objectives at once. First, and most obviously, the inclusion of a passive needle safety system drastically reduces the chance of a needlestick injury, along with all of the subsequent anxiety, trauma and cost that can come with it. Audible, tactile and visual cues inform the user when the drug has been fully delivered and the needle has been safely retracted, providing comfort and clarity during a stressful procedure. The post-injection format with the needle fully protected makes the disposal of sharps waste safer and easier. In addition, the Dual Chamber system enables an easier and more intuitive reconstitution or sequential delivery process, without adding multiple packages, steps, waste and training, thereby promoting safe, accurate and successful administration.
Beyond the environmental and societal benefits directly associated with the Credence Companion and Dual Chamber, these systems can also enhance the value of related device formats. Typically, self-injecting patients prefer the flexibility of choosing between a standalone syringe-based system, where they have more control of the injection, and a dose-assisting device such as an autoinjector. And, with sustainability in mind, it is highly desirable for the autoinjector to be a reusable device, with a disposable prefilled syringe loaded by the user.
“The small footprint of the Companion and Dual Chamber systems allows for a reusable autoinjector with a comfortable and efficient form factor, which has both usability and environmental advantages.”
The small footprint of the Companion and Dual Chamber systems allows for a reusable autoinjector with a comfortable and efficient form factor, which has both usability and environmental advantages. The needle retraction into the plunger rod ensures that the disposable element is small, safe and easily removed by the user. As such, Credence technology enables the implementation of reusable autoinjectors and minimises waste while improving usability and safety for the user. This is a clear example of how ESG factors frequently overlap, so taking a holistic approach to both social and environmental considerations is always advantageous. Credence’s Innovation Without Change approach once again demonstrates the way to make sustainability an opportunity.
CONCLUSION
The pharma industry is not an exception to the global push towards sustainability, even while regulation and patient safety concerns pose unique challenges. To make sustainability work for the industry, it must be seen as more than an obligation or a compromise – a positive business case must be made for it, with sustainability going hand in hand with reduced costs and improved outcomes. To achieve this, it is crucial to adopt an Innovation Without Change approach to implementing more sustainable products and practices.
Credence is showing the way forward in this effort, with the Credence Companion and Dual Chamber systems being ideal exemplars for how innovative emerging companies can forge ahead and provide an implementable pathway for larger, more established companies to adopt. By reducing material usage and minimising the need to alter existing infrastructure, the pharma industry can achieve sustainability gains and cost reductions across primary and secondary packaging, logistics and manufacturing.
Furthermore, by expanding the sustainability conversation to consider both the environmental and social aspects of ESG – reducing the burden of treatment on patients and healthcare systems as well as reducing the environmental impact – the industry can ensure that the steps taken towards sustainability are lasting and effective. With its Companion and Dual Chamber platforms, Credence has demonstrated that sustainability need not be a burden, but can in fact be the driver behind products that are better for users, better for the environment and better for the pharma industry as a whole.
REFERENCES
- “The increase in appetite for obesity drugs”. J.P. Morgan, Nov 2023.
- “Global Self Injection Device Market Size, Share, and COVID-19 Impact Analysis, By Product, By Usability, By Application, and By Region, Analysis and Forecast 2023 – 2033”. Spherical Insights, Mar 2024.
- “Prefilled Syringes Market Size, Share, & Trends by Type, Material, Design, Application, & Region – Global Forecast to 2030)”. Markets and Markets, Jun 2024.
- “The evolving cold chain needs of the pharmaceutical industry”. Maersk, Apr 2024.
- “Pharmaceutical Secondary Packaging Market”. Roots Analysis, Mar 2022.
- “Plastic Credits: How Much Do They Cost? Factors That Influence The Price Of Plastic Credit”. ALLCOT Trading, Dec 2022.

