To Issue 134
Citation: Schneider A, Müller P, “Paving the Way to Integrated Device-Digital Solutions”. ONdrugDelivery, Issue 134 (Jun 2022), pp 46–50.
Andreas Schneider and Philippe Müller discuss the potential benefits and challenges of implementing connected device-digital solutions within the healthcare sector to improve the treatment of chronic conditions.
“Since 2017, the US FDA alone has approved more than 40 digital therapeutics and health apps addressing chronic conditions, including diabetes, back pain, ADHD and asthma.”
“Seems like you have just missed another dose, Adam, let’s discuss how to best fit your injections into your daily routine. Surely, I can help you with that.” The warm and empathic voice gently gives the necessary instructions to Adam, who has suffered from atopic dermatitis for years and has been prescribed a new biologics treatment that must be self-injected every two weeks. Surprisingly, however, this voice does not come from his attending physician, but from an artificial intelligence engine that issues its commands straight from the speakers of his smartphone. It is barely recognisable as a robotic voice.
The algorithm that fuels the digital solution recognised that Adam had repeatedly missed his dose and identified him as a patient at risk of prematurely stopping self-injection and not achieving his treatment goals due to medication non-adherence. If Adam continues like this, he will suffer from debilitating pruritus, disturbed sleep and mental distress. As with up to half of the more than 26 million other Americans diagnosed with atopic dermatitis, these effects will also negatively impact Adam’s lifestyle, work and education. These are effects that Adam is well aware of in principle, but which are only marginally present at this time.1–6
RECENT ADVANCES WITH DIGITAL THERAPEUTICS
Integrated device-digital solutions to support behavioural change and empower patients diagnosed with chronic conditions to manage and improve their symptoms are currently gaining traction within the healthcare sector. Since 2017, the US FDA alone has approved more than 40 digital therapeutics and health apps addressing chronic conditions, including diabetes, back pain, ADHD and asthma.
These solutions encourage patients to engage with their specific disease and symptoms to optimise their treatment pathway and outcomes (Figure 1). Moreover, new pathways are presently being established for digital health apps to be paid for through existing healthcare systems. For example, in Germany, 12 digital solutions have provided sufficient clinical evidence to be permanently approved and reimbursed.11

Figure 1: Added value of digital therapeutics per exemplar chronic disease areas.7–10
“An integrated device-digital solution may be available on prescription and rolled out in conjunction with the medication to improve medication adherence, preventive measures and programme engagement, as well as to deliver improvements in health outcomes.”
TOWARDS INTEGRATED DEVICE-DIGITAL SOLUTIONS
Consider Adam, who may require a device-digital solution that provides sustained and holistic support to achieve effective disease control and comfort. Such a solution should fully support him in managing, and ultimately improving, his medication intake and disease symptoms. Ideally, the digital health app is paired with a connected drug delivery device that seamlessly feeds data into the digital system. The connected device should require minimal user interaction, passively log injection events and support Adam through the most critical use steps. After being integrated with other data sources and enriched with electronic patient-reported outcomes, the data aggregated from such a system can be presented to the attending physician to enable them to manage medication and evaluate treatment for their patients efficiently and effectively.
Such an integrated device-digital solution, as illustrated in Figure 2, may be available on prescription and rolled out in conjunction with the medication to improve medication adherence, preventive measures and programme engagement, as well as to deliver improvements in health outcomes. In addition, the anonymised connected device and patient-reported outcomes data can provide valuable real-world evidence for pharma companies, including for medication adherence, therapy persistence and quality of life. These insights might then be used as inputs to feed predictive algorithms for treatment evaluation and further research activities.
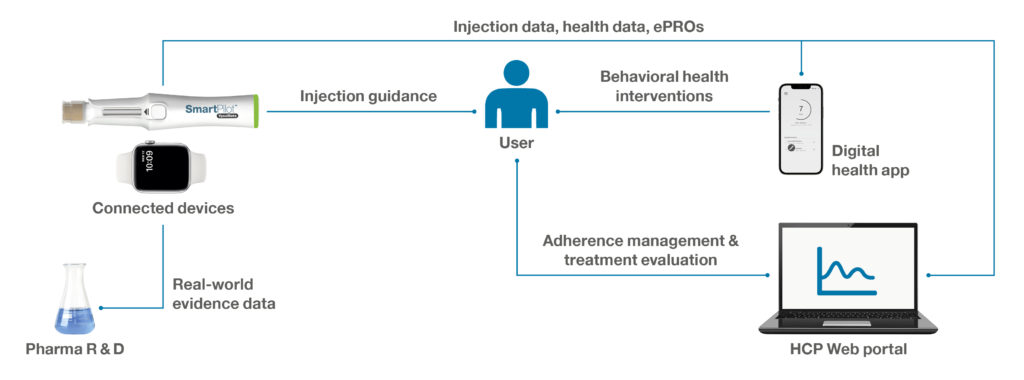
Figure 2: Illustration of an integrated device-digital solution to support behavioural change and empower patients to manage adherence and disease symptoms.
Regardless of the focus disease area, significant efforts are being made to improve treatment adherence. Medication non-adherence is a major problem across the healthcare sector that affects treatment outcomes and imposes significant costs on healthcare systems. In fact, 50–60% of patients being treated for chronic conditions either miss doses, take the wrong doses or discontinue treatment within the first year.12 Non-adherence to medication results from patient-specific interactions within five sets of factors (Figure 3):
- Disease-related factors
- Socio-economic factors
- Health-system-related factors
- Therapy-related factors
- Patient-related factors.9
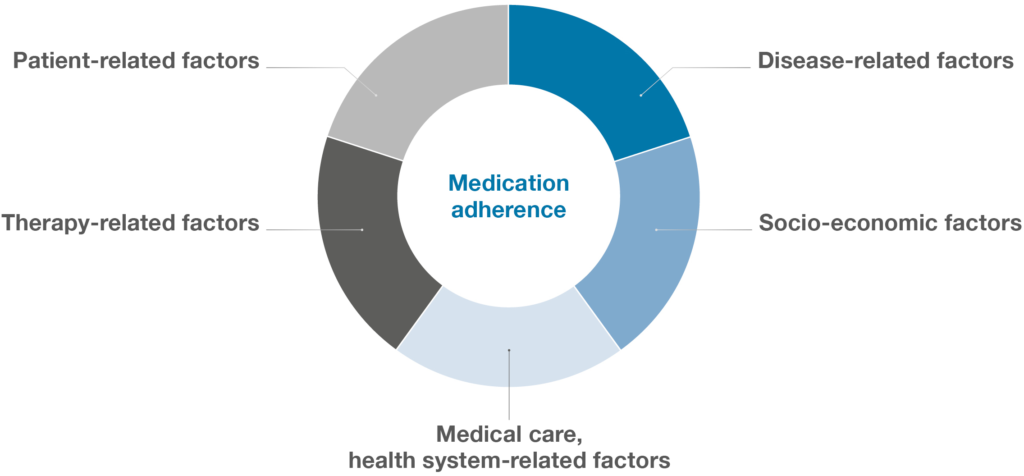
Figure 3: The multidimensional nature of medication adherence.
NEED TO ADDRESS SPECIFIC ADHERENCE BARRIERS
Consequently, any device-digital solution aimed at improving medication adherence must address specific barriers and should be tailored to the specific needs of the patient and their therapy context.13 A device-digital solution boosts medication adherence by adapting to the patient’s needs – not the other way around. This observation has been echoed by the pharmaceutical industry, which has shifted its attention from the development of generic adherence management systems to the development of personalised and therapy-specific device-digital solutions.
Modern designs aim to improve medication adherence by adapting the provision of support, including type, timing and intensity, to an individual’s changing status and contexts over time. The underlying goal is to deliver support when the patient most needs it and is most likely to be receptive – the point at which they are most likely to change their behaviour.14 To this end, smart connected drug delivery devices are key because they can act on both the timeliness and adaptive nature of these interventions by monitoring the dynamics of an individual’s state and context in real time (Figure 4).
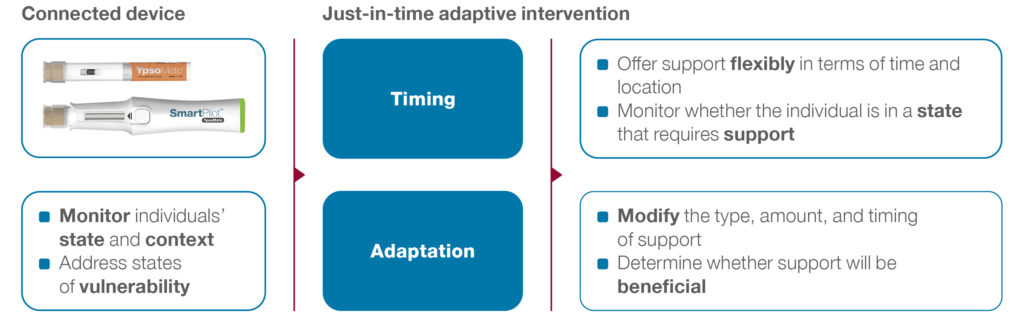
Figure 4: Smart connected devices as a basis for personalised digital health solutions.
“Ypsomed is advancing a broad portfolio of connected injection devices that can be structured both into connected add-ons for its proven mechanical self-injection devices and into autoinjectors with integrated connectivity.”
Consider Adam, who may have concerns relating to the use of his drug delivery device. Should his connected drug delivery device detect a use error, it may trigger a digital health intervention that is carefully tailored to the type of use error and Adam’s specific user profile. In addition, the connected device reports the use error in nearly real time, which allows it to trigger an intervention at the point when the error happens, which is when Adam will be most receptive to tips on how to improve his behaviour.
Thus, data from connected devices serve as an important input for digital therapy management solutions, enabling effective behavioural health interventions in terms of both content and timing. Such functionality can help patients to more effectively manage their injection regimens and set their expectations regarding their treatment that may, in turn, propel more successful treatment outcomes.
ADVANCING A BROAD PORTFOLIO OF CONNECTED DEVICES
Therefore, Ypsomed is advancing a broad portfolio of connected injection devices that can be structured both into connected add-ons for its proven mechanical self-injection devices and into autoinjectors with integrated connectivity (Figure 5). First, SmartPilot is the reusable connected add-on for the two-step autoinjector YpsoMate. SmartPilot captures injection events, detects use errors and provides comprehensive real-time injection support, including drug authentication at point of use and step-by-step guidance. Second, YpsoMate On is a prefilled autoinjector with integrated connectivity that can automatically log injections based on advanced proximity measuring protocols, similar to the working principle of covid-19 contact tracing apps.
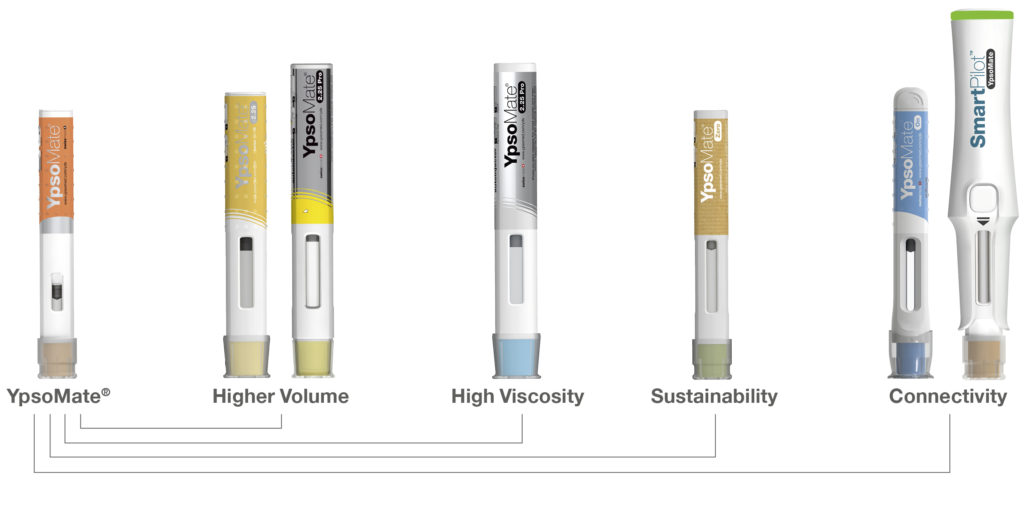
Figure 5: Evolution of the Ypsomed autoinjector portfolio.
While YpsoMate On provides a narrower feature set compared with SmartPilot, it retains YpsoMate’s proven two-step device handling and enables automated data capture. Moreover, the device includes LED-based visual feedback to signal an ongoing injection and completion of the injection, including the hold time. The device choice ultimately depends on the intended use of the overall system, as well as the specific user population, injection frequency and other disease-and therapy-specific aspects.
CONCLUDING REMARKS
There is growing evidence of the benefits of device-digital solutions across various disease areas. However, to ensure broad market acceptance, the industry must overcome the challenges associated with the implementation of such device-digital solutions. As Norbert Lauber (Director Autoinjectors & Pump Systems, Novartis Pharma) aptly put it at the SMi Wearable Injectors and Connected Devices conference in 2021, “Adding connectivity expands the combined product into a complex collection of components”.15
As such, close collaboration between device manufacturers and digital therapy management providers is needed both to lower the complexities of implementing a digital system and to design effective justin- time interventions tailored to the specific needs of individuals. Joining forces between digital and device solution providers will be a winning formula to deliver substantial value-add for the pharmaceutical industry, patients and the entire healthcare system.
REFERENCES
- Shaw ES et al, “Eczema Prevalence in the United States: Data from the 2003 National Survey of Children’s Health”. J Invest Dermatol, 2011, Vol 131(1), pp 67–73.
- Fuxench ZCC et al, “Atopic Dermatitis in America Study: A Cross-Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population”. J Invest Dermatol, 2019, Vol 139(3), pp 583–590.
- Weidinger S et al, “Atopic Dermatitis”. Nat Rev Dis Primers, 2018, Vol 4(1), p 1.
- Silverberg JI et al, “Patient Burden and Quality of Life in Atopic Dermatitis in US Adults: A Population-Based Cross-Sectional Study”. Ann Allergy Asthma Immunol, 2018, Vol 121(3), pp 340–347.
- Chung J, Simpson EL, “The Socioeconomics of Atopic Dermatitis”. Ann Allergy Asthma Immunol, 2019, Vol 122(4), pp 360–366.
- Holm EA, Esmann S, Jemec GBE, “The Handicap Caused by Atopic Dermatitis – Sick Leave and Job Avoidance”. J Eur Dermatol Venereol, 2006, Vol 20(3), pp 255–259.
- Aapro M et al, “Digital Health for Optimal Supportive Care in Oncology: Benefits, Limits, and FuturePerspectives”. Support Care Cancer, 2020, Vol 28(10), pp 4589–4612.
- Basch E et al, “Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment”. JAMA, 2017, Vol 318(2), pp 197–198.
- Solomon DH, Rudin RS, “Digital Health Technologies: Opportunities and Challenges in Rheumatology”. Nat Rev Rheumatol, 2020, Vol 16(9), pp 525–535.
- Thorgeirsson T et al, “Randomized Trial for Weight Loss Using a Digital Therapeutic Application”. J Diabetes Sci Technol, 2021, 19322968211000815.
- “Some Health Apps Are Able Not Just to Diagnose Diseases, But Also to Treat Them”. The Economist, May 2022.
- Hichborn J et al, “Improving Patient Adherence Through Data-Driven Insights”. McKinsey & Company, Dec 2018.
- “Adherence to Long-Term Therapies: Evidence for Action” (Sabaté E, ed). WHO, 2003.
- Nahum-Shani I et al, “Just-in-Time Adaptive Interventions (JITAIs) in Mobile Health: Key Components and Design Principles for Ongoing Health Behavior Support”. Ann Behav Med, 2018, Vol 52(6), pp 446–462.
- Lauber N, “Wearable Injectors and Digital Applications”. Presentation at SMi Wearable Injectors and Connected Devices conference, 2021.

