To Issue 152
Citation: Thueer T, Muenzer C, Schlenker I, “PiccoJect – Using Human Factors to Fulfil Patients’ Need for Injection Confidence”. ONdrugDelivery, Issue 152 (Oct 2023), pp 44–48.
Thomas Thueer, Chris Muenzer and Ines Schlenker discuss the development of the enhanced usability features of PiccoJect, Haselmeier‘s novel autoinjector.
INTRODUCTION
Injection, especially self-injection, can be a daunting prospect for patients. It is therefore critical for injection devices to emphasise features that enhance usability and instil confidence in their users. To achieve this, human factors studies are a crucial element of the development process. By undertaking such studies, drug delivery device developers can access invaluable feedback from patients on their needs, preferences and desires, which can then be incorporated into their device design to improve its usability and reduce the risk of use errors.
“PiccoJect was developed according to the guiding principle of ‘excellence through simplicity’, which is embodied throughout its design.”
In recent years, device developers have refined the techniques for establishing what patients will accept and incorporating real user feedback into the device design. Regulators, too, have recognised the value of human factors studies, with many regulatory agencies now expecting human factors studies to be included as part of any new combination product submission.
PICCOJECT – USABILITY AT THE FOREFRONT OF DESIGN
To meet the needs of patients, Haselmeier has developed PiccoJect – a compact, fully-featured two-step autoinjector platform for subcutaneous drug delivery (Figure 1) that can be easily adapted to customers’ needs. PiccoJect was developed according to the guiding principle of “excellence through simplicity”, which is embodied throughout its design.

Figure 1: Haselmeier’s PiccoJect is a compact, fully-featured two-step autoinjector designed for subcutaneous delivery of drug products, compatible with any standard 1 mL long or 2.25 mL PFS.
Human factors and patient-centricity have played a key role in the development of PiccoJect, with patient needs placed firmly at the heart of its design. PiccoJect’s design has been also recognised for excellence by the industry, winning the Good Design Award 2022 and the Red Dot Award 2023. To maximise usability, the device has a flat, compact design that improves the device’s ergonomics for a broad patient population, as well as keeping it small and easily portable. PiccoJect also features a large wrap-around viewing window for patients to observe the progress of their injection, as well as a coloured status indicator to provide clear information about its use status (Figure 2). Furthermore, PiccoJect’s shape and internal layout enable the use of larger-diameter springs, allowing Haselmeier to better optimise the spring force to match the injection time to the drug properties.
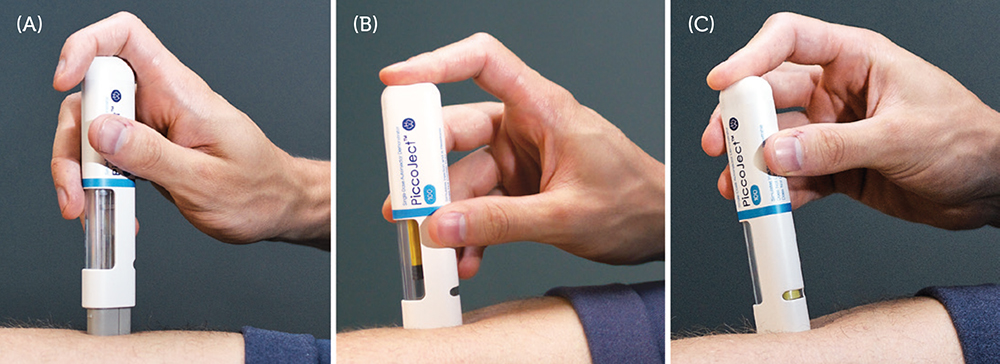
Figure 2: (A) PiccoJect directly before injection with the cap already removed. (B) PiccoJect during injection – the yellow plunger moves down in the large wrap-around drug window. (C) PiccoJect immediately after the injection is finished – the status indicator shows that injection is completed.
“Participants noted that PiccoJect offered them greater usability, in particular due to the comfort and security of PiccoJect’s wide cross-section (18 out of 24) and its large viewing window (20 out of 24).”
PiccoJect has been designed as a full-service platform, with support from early-phase combination product development through design verification up to final assembly, packaging, labelling and serialisation. In keeping with the guiding principle of “excellence through simplicity”, PiccoJect has an extremely low part count, consisting of only eight parts in total, which significantly lessens the challenges associated with manufacturing and scale-up. Also, in accordance with this principle, PiccoJect is compatible with any standard 1 mL long or 2.25 mL prefilled syringe (PFS).
In keeping with the industry-wide push towards greater sustainability, PiccoJect has been developed using plastic materials from sustainable feedstocks and low-carbon electricity during manufacture. Additionally, PiccoJect’s design has been future-proofed, with multiple connectivity options in development.

Figure 3: PiccoJect has a number of features specifically developed to provide patients with confidence in their treatment, including excellent visibility of the injection’s progress through its large wrap-around viewing window, and audible clicks at the start and end of injection to ensure that the device is held in place until the full dose has been injected.
UNDERSTANDING WHAT PATIENTS WANT FROM PICCOJECT
Haselmeier partnered with DCA to ensure that the lived experience of patients was kept at the heart of PiccoJect’s design. The combined development team has extensive design experience developing injection devices, covering a wide range of therapies. This breadth of experience was invaluable for conducting a series of human factors studies on PiccoJect, both in formal user studies and more free-form conversations. In Haselmeier’s experience, patients prioritise one overarching quality in a drug delivery device – confidence (Figure 3). PiccoJect’s features are specifically designed to provide patients with confidence throughout the injection process, helping them to physically interact with the device and to understand:
- When to use the device
- That the drug inside is safe to inject
- How to use the device
- That the device is working as intended
- That delivery has been successful.
FIRST HUMAN FACTORS STUDY
After initial prototyping, the first study investigated five autoinjector models, covering both 1 and 2.25 mL variants. Three of the functional models were PiccoJect prototypes and two were representative of an existing marketed autoinjector as a benchmark. All of the models were visually representative and contained no drugs or needles, with the intent being to assess patient responses to a simulated injection, including handling and visual feedback features.
This study set out to explore potential sources of use errors and handling issues, as well as to investigate patient preferences, which could then be used to inform the next phases of PiccoJect’s design. Across a total of 24 participants, the study contained an even split of experienced and injection-naive users aged between 25 and 67 years. No training or assistance was provided except for a simplified instructions for use (IFU), with half of the participants performing self-injections and half performing the injection on a mannequin. All users performed an injection with each of the five autoinjector models. PiccoJect matched the marketed device in terms of task performance.
To investigate user preferences, participants were initially asked for their first impressions of the devices before handling them. For this purpose, a 2D image was shown to the participants. After performing the injection, the participants were asked again for their feedback. The initial impressions generally favoured PiccoJect, with a majority of participants naming one of the PiccoJect models as the one that they were instinctively drawn to, as they found it the most attractive and the easiest to use.
“The mean score across all participants was 6.6 (between ‘Happy’ and ‘Very Happy’), with 23 out of 24 participants giving either a ‘Happy’ or ‘Very Happy’ rating.”
Notably, a shorter model of PiccoJect included in the study proved polarising, with study participants frequently taking either a strong like or a strong dislike to the smaller size. Interestingly, a number of participants who chose it as their favourite of the 2D images then revised their selection to one of the standard-length PiccoJect models after handling and using them.
After completing the study, participants were asked which of the five models was their favourite. Overall, 92% of participants (22 out of 24) chose one of the PiccoJect models. Participants noted that PiccoJect offered them greater usability, in particular due to the comfort and security of PiccoJect’s wide cross-section (18 out of 24) and its large viewing window (20 out of 24). This was highly encouraging feedback for the design team, as it demonstrated that PiccoJect’s usability features successfully offered participants the confidence they desired.
SECOND HUMAN FACTORS STUDY
The second study was conducted once a fully functional prototype of PiccoJect had been developed, with the aim of confirming the value of PiccoJect’s advanced usability features using these more realistic devices. As with the first study, the second study had 24 participants, all adults, with a mixture of experienced and injection-naive users, half of whom had dexterity issues. Again, participants were provided with no instruction or assistance beyond a simplified IFU. Across a total of 96 injections, the task performance matched expectations for a two-step autoinjector and the findings of the previous study.
After completing the study, participants were asked to rate PiccoJect on a Likert scale (1–7) to indicate how willing they would be to use the device on a daily basis. The mean score across all participants was 6.6 (between “Happy” and “Very Happy”), with 23 out of 24 participants giving either a “Happy” or “Very Happy” rating. All 12 experienced users stated that they would be willing to replace their current injection device with PiccoJect.
THIRD HUMAN FACTORS STUDY
The most recent study further demonstrated the value of PiccoJect’s usability features using devices manufactured with components from the commercial injection moulds. The third study followed the same format as the first and second, with 24 adult participants, of whom some were experienced with injection devices and some were injection-naive users. Again, several of the participants had dexterity issues, including at least one with fairly severe impairments. Participants were asked to rank individual features on a Likert scale, and nearly all features were scored between six and seven on average (Figure 4).
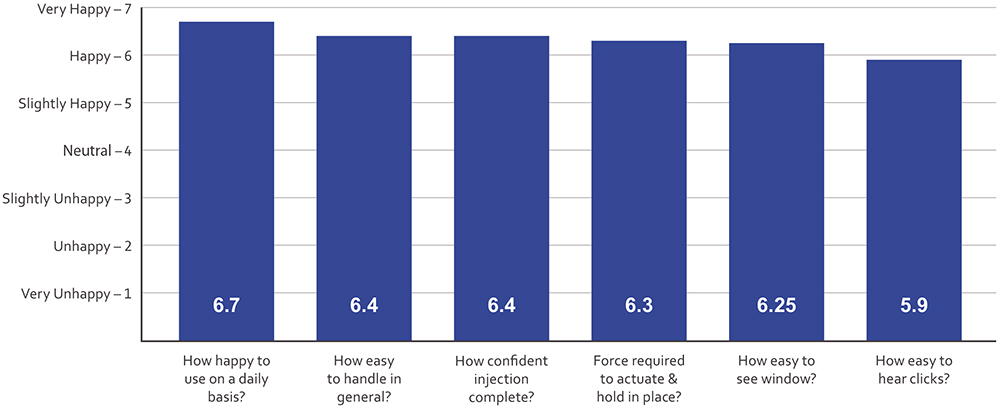
Figure 4: Summary of the third human factors study conducted in 2023. Users provided subjective feedback on different usability aspects of PiccoJect on a Likert scale, with seven being highest and one being lowest. Nearly all aspects achieved ratings between six and seven.
One study participant with severe dexterity impairment (Cochin score of 63) stated “I really like the shape – it’s not like anything I’ve used before. How natural it feels.” They went on to say that “I think I might be able to use this myself, and unsupervised, which is a really massive thing for me”. This shows the potential significance of the confidence and independence that a feature as simple as PiccoJect’s flat cross-section can offer patients with dexterity issues.
CONCLUSION
When designing an injection device, it is imperative to pay close attention to the needs of patients, incorporating their desires and needs into the design as early as possible. Formative human factors studies, conducted throughout the development cycle, can allow developers to keep the patient at the heart of the design, resulting in an injection device with usability features that can instil patients with confidence. Firmly believing in this principle, Haselmeier has developed the PiccoJect autoinjector, designed with the guiding principle “excellence through simplicity” at its core. Across three formative usability studies, participants were highly positive about PiccoJect. In particular, patients valued its usability features, including the large wraparound viewing window, flat cross-section and clear feedback, with nearly all study participants stating that they would be happy to use PiccoJect on a daily basis to inject their medications (Figure 5).
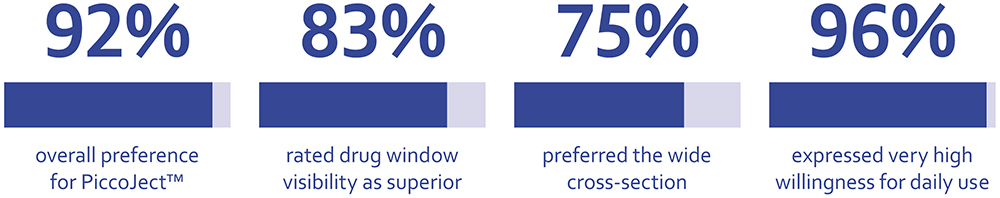
Figure 5: Summary of patients’ preference for PiccoJect from across the first two human factors studies.
With simplicity set as the core requirement, PiccoJect is easy to manufacture, adaptable to the specific needs of a given drug and compatible with all standard 1 mL long and 2.25 mL PFSs. With PiccoJect, Haselmeier offers a fully featured device platform with a strong focus on usability that is favoured by users, who have provided very positive feedback in several human factors studies.
Learn more about Haselmeier’s PiccoJect by visiting: haselmeier.com/en/piccoject.